Reduction of surface treatment time by combination of citric acid and ascorbic acid while restoring shear bond strength of metal brackets bonded to bleached enamel: a pilot study
- PMID: 38867181
- PMCID: PMC11167946
- DOI: 10.1186/s12903-024-04424-1
Reduction of surface treatment time by combination of citric acid and ascorbic acid while restoring shear bond strength of metal brackets bonded to bleached enamel: a pilot study
Erratum in
-
Correction: Reduction of surface treatment time by combination of citric acid and ascorbic acid while restoring shear bond strength of metal brackets bonded to bleached enamel: a pilot study.BMC Oral Health. 2024 Aug 1;24(1):873. doi: 10.1186/s12903-024-04648-1. BMC Oral Health. 2024. PMID: 39090701 Free PMC article. No abstract available.
Abstract
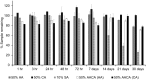
Background: To investigate the effect of a 50% ascorbic acid with 50% citric acid solution on the immediate shear bond strength (SBS) of metallic brackets after tooth bleaching. The enamel etching pattern and the required quantity of these combined acids as antioxidants following 35% hydrogen peroxide (HP) bleaching were also determined.
Methods: The stability of the solution at room temperature was assessed at various time intervals. Fifty teeth were randomly divided into five groups: non-bleached (G1), bleached then acid etched (G2), bleached followed by a 10-minute treatment with 10% sodium ascorbate and acid etched (G3), 5-minute treatment with 50% ascorbic acid (G4), and 5-minute treatment with a combination of 50% ascorbic acid and 50% citric acid (G5). Groups G2, G3, G4 and G5 were bleached by 35% HP gel for a total of 32 min. Acid etching in groups G1, G2, and G3 was performed using 37% phosphoric acid (Ormco®, Orange, CA, USA) for 15 s. In all groups, metal brackets were immediately bonded using Transbond™ XT primer and Transbond™ PLUS adhesive, with light curing for 40 s. The SBS was tested with a universal testing machine, and statistical analysis was conducted using one-way ANOVA followed by Tukey's HSD test. The level of significance was set at p < 0.05 for all statistical tests.
Results: Stability tests demonstrated that the combined acids remained effective for up to 21 days. Group G5 significantly increased the SBS of bleached teeth to the level of G1 (p < 0.05), while G3 did not achieve the same increase in SBS (p > 0.05). SEM analysis revealed enamel etching patterns similar to those of both control groups (G1 and G2). Kinetic studies at 6 min indicated that the antioxidation in G5 reacted 0.2 mmole lower than in G3 and G4.
Conclusion: 5-minute application of the combined acids enhanced the SBS of bleached teeth comparable to unbleached teeth. The combined acids remain stable over two weeks, presenting a time-efficient, single-step solution for antioxidant application and enamel etching in orthodontic bracket bonding.
Keywords: Antioxidant; Ascorbic acid; Bleaching; Citric acid; Combined of citric acid and ascorbic acid; Shear bond strength and brackets.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Initial shear bond strength of orthodontic brackets bonded to bleached teeth with a self-etching adhesive system.Quintessence Int. 2012 May;43(5):e60-6. Quintessence Int. 2012. PMID: 22536597 Clinical Trial.
-
Effect of different surface treatment on the shear bond strength of metal brackets to bleached and desensitized enamel.Int Orthod. 2019 Mar;17(1):73-79. doi: 10.1016/j.ortho.2019.01.008. Epub 2019 Feb 13. Int Orthod. 2019. PMID: 30770334
-
Influence of microhybrid resin and etching times on bleached enamel for the bonding of ceramic brackets.Braz Oral Res. 2013 Mar-Apr;27(2):142-8. doi: 10.1590/s1806-83242013000100019. Braz Oral Res. 2013. PMID: 23538425
-
Effect of an antioxidizing agent on the shear bond strength of brackets bonded to bleached human enamel.Am J Orthod Dentofacial Orthop. 2006 Feb;129(2):266-72. doi: 10.1016/j.ajodo.2004.03.043. Am J Orthod Dentofacial Orthop. 2006. PMID: 16473720
-
Effects of different types of laser etching versus phosphoric acid etching on shear bond strength of metal brackets to human enamel: A systematic review and meta-analysis of in vitro studies.Int Orthod. 2020 Dec;18(4):673-683. doi: 10.1016/j.ortho.2020.10.001. Epub 2020 Nov 2. Int Orthod. 2020. PMID: 33144060
Cited by
-
Correction: Reduction of surface treatment time by combination of citric acid and ascorbic acid while restoring shear bond strength of metal brackets bonded to bleached enamel: a pilot study.BMC Oral Health. 2024 Aug 1;24(1):873. doi: 10.1186/s12903-024-04648-1. BMC Oral Health. 2024. PMID: 39090701 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

