Dynamics of pulmonary mucosal cytotoxic CD8 T-cells in people living with HIV under suppressive antiretroviral therapy
- PMID: 38867225
- PMCID: PMC11170847
- DOI: 10.1186/s12931-024-02859-2
Dynamics of pulmonary mucosal cytotoxic CD8 T-cells in people living with HIV under suppressive antiretroviral therapy
Abstract
Background: Despite the success of antiretroviral therapy (ART), people living with HIV (PLWH) suffer from a high burden of pulmonary diseases, even after accounting for their smoking status. Cytotoxic CD8 T-cells are likely implicated in this phenomenon and may act as a double-edged sword. While being essential in viral infection control, their hyperactivation can also contribute to lung mucosal tissue damage. The effects of HIV and smoking on pulmonary mucosal CD8 T-cell dynamics has been a neglected area of research, which we address herein.
Methods: Bronchoalveolar lavage (BAL) fluid were obtained from ART-treated PLWH (median duration of supressed viral load: 9 years; smokers: n = 14; non-smokers: n = 21) and HIV-uninfected controls (smokers: n = 11; non-smokers: n = 20) without any respiratory symptoms or active infection. Lymphocytes were isolated and CD8 T-cell subsets and homing markers were characterized by multiparametric flow cytometry.
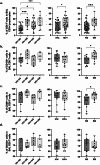
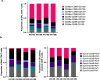
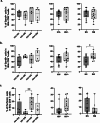
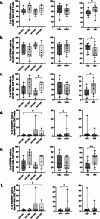
Results: Both smoking and HIV infection were independently associated with a significant increase in frequencies of total pulmonary mucosal CD8 T-cell. BAL CD8 T-cells were primarily CD69 + expressing CD103 and/or CD49a, at least one of the two granzymes (GzmA/GzmB), and little Perforin. Higher expression levels of CD103, CD69, and GzmB were observed in smokers versus non-smokers. The ex vivo phenotype of GzmA + and GzmB + cells revealed increased expression of CD103 and CXCR6 in smokers, while PLWH displayed elevated levels of CX3CR1 compared to controls.
Conclusion: Smoking and HIV could promote cytotoxic CD8 T-cell retention in small airways through different mechanisms. Smoking likely increases recruitment and retention of GzmB + CD8 Trm via CXCR6 and CD103. Heightened CX3CR1 expression could be associated with CD8 non-Trm recruitment from the periphery in PLWH.
Keywords: CD8 T-cells; HIV; Lung; People living with HIV (PLWH); Pulmonary immunity; Smoking; Tissue resident; Trm.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Peculiar Phenotypic and Cytotoxic Features of Pulmonary Mucosal CD8 T Cells in People Living with HIV Receiving Long-Term Antiretroviral Therapy.J Immunol. 2021 Feb 1;206(3):641-651. doi: 10.4049/jimmunol.2000916. Epub 2020 Dec 14. J Immunol. 2021. PMID: 33318292
-
HIV Infection and Persistence in Pulmonary Mucosal Double Negative T Cells In Vivo.J Virol. 2020 Nov 23;94(24):e01788-20. doi: 10.1128/JVI.01788-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967958 Free PMC article.
-
Smoking and Human Immunodeficiency Virus 1 Infection Promote Retention of CD8+ T Cells in the Airway Mucosa.Am J Respir Cell Mol Biol. 2021 Nov;65(5):513-520. doi: 10.1165/rcmb.2021-0168OC. Am J Respir Cell Mol Biol. 2021. PMID: 34166603 Free PMC article.
-
[Deep lung--cellular reaction to HIV].Rev Port Pneumol. 2007 Mar-Apr;13(2):175-212. Rev Port Pneumol. 2007. PMID: 17492233 Review. Portuguese.
-
The Emerging Role of CD8+ Tissue Resident Memory T (TRM) Cells in Antitumor Immunity: A Unique Functional Contribution of the CD103 Integrin.Front Immunol. 2018 Aug 15;9:1904. doi: 10.3389/fimmu.2018.01904. eCollection 2018. Front Immunol. 2018. PMID: 30158938 Free PMC article. Review.
Cited by
-
Immune Alterations and Viral Reservoir Atlas in SIV-Infected Chinese Rhesus Macaques.Infect Dis Rep. 2025 Feb 6;17(1):12. doi: 10.3390/idr17010012. Infect Dis Rep. 2025. PMID: 39997464 Free PMC article. Review.
-
B cell deficiency induces cytotoxic memory CD8+ T cells during influenza-associated bacterial pneumonia.J Clin Invest. 2025 Jun 10;135(16):e188342. doi: 10.1172/JCI188342. eCollection 2025 Aug 15. J Clin Invest. 2025. PMID: 40493422 Free PMC article.
-
CD4+T and CD8+T cells profile in lung inflammation and fibrosis: targets and potential therapeutic drugs.Front Immunol. 2025 May 9;16:1562892. doi: 10.3389/fimmu.2025.1562892. eCollection 2025. Front Immunol. 2025. PMID: 40433386 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

