Secretion of the cytoplasmic and high molecular weight β-galactosidase of Paenibacillus wynnii with Bacillus subtilis
- PMID: 38867249
- PMCID: PMC11167759
- DOI: 10.1186/s12934-024-02445-7
Secretion of the cytoplasmic and high molecular weight β-galactosidase of Paenibacillus wynnii with Bacillus subtilis
Abstract
Background: The gram-positive bacterium Bacillus subtilis is widely used for industrial enzyme production. Its ability to secrete a wide range of enzymes into the extracellular medium especially facilitates downstream processing since cell disruption is avoided. Although various heterologous enzymes have been successfully secreted with B. subtilis, the secretion of cytoplasmic enzymes with high molecular weight is challenging. Only a few studies report on the secretion of cytoplasmic enzymes with a molecular weight > 100 kDa.
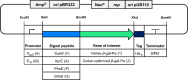
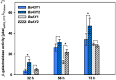
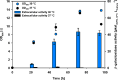
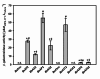
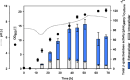
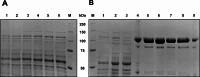
Results: In this study, the cytoplasmic and 120 kDa β-galactosidase of Paenibacillus wynnii (β-gal-Pw) was expressed and secreted with B. subtilis SCK6. Different strategies were focused on to identify the best secretion conditions. Tailormade codon-optimization of the β-gal-Pw gene led to an increase in extracellular β-gal-Pw production. Consequently, the optimized gene was used to test four signal peptides and two promoters in different combinations. Differences in extracellular β-gal-Pw activity between the recombinant B. subtilis strains were observed with the successful secretion being highly dependent on the specific combination of promoter and signal peptide used. Interestingly, signal peptides of both the general secretory- and the twin-arginine translocation pathway mediated secretion. The highest extracellular activity of 55.2 ± 6 µkat/Lculture was reached when secretion was mediated by the PhoD signal peptide and expression was controlled by the PAprE promoter. Production of extracellular β-gal-Pw was further enhanced 1.4-fold in a bioreactor cultivation to 77.5 ± 10 µkat/Lculture with secretion efficiencies of more than 80%.
Conclusion: For the first time, the β-gal-Pw was efficiently secreted with B. subtilis SCK6, demonstrating the potential of this strain for secretory production of cytoplasmic, high molecular weight enzymes.
Keywords: Bacillus subtilis; β-galactosidase; Bioreactor; Protein secretion; Recombinant enzyme production.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Recombinant production of Paenibacillus wynnii β-galactosidase with Komagataella phaffii.Microb Cell Fact. 2024 Oct 5;23(1):263. doi: 10.1186/s12934-024-02544-5. Microb Cell Fact. 2024. PMID: 39367390 Free PMC article.
-
Efficient Secretion of the β-Galactosidase Bgal1-3 via both Tat-Dependent and Tat-Independent Pathways in Bacillus subtilis.J Agric Food Chem. 2016 Jul 20;64(28):5708-16. doi: 10.1021/acs.jafc.6b01735. Epub 2016 Jul 12. J Agric Food Chem. 2016. PMID: 27380825
-
Extracellular secretion in Bacillus subtilis of a cytoplasmic thermostable beta-galactosidase from Geobacillus stearothermophilus.J Dairy Sci. 2010 Jul;93(7):2838-45. doi: 10.3168/jds.2009-2864. J Dairy Sci. 2010. PMID: 20630200
-
Exploitation of Bacillus subtilis as a robust workhorse for production of heterologous proteins and beyond.World J Microbiol Biotechnol. 2018 Sep 10;34(10):145. doi: 10.1007/s11274-018-2531-7. World J Microbiol Biotechnol. 2018. PMID: 30203131 Review.
-
Bacillus subtilis: current and future modification strategies as a protein secreting factory.World J Microbiol Biotechnol. 2024 May 9;40(6):195. doi: 10.1007/s11274-024-03997-x. World J Microbiol Biotechnol. 2024. PMID: 38722426 Review.
Cited by
-
Recombinant production of Paenibacillus wynnii β-galactosidase with Komagataella phaffii.Microb Cell Fact. 2024 Oct 5;23(1):263. doi: 10.1186/s12934-024-02544-5. Microb Cell Fact. 2024. PMID: 39367390 Free PMC article.
-
Importance of the 5' untranslated region for recombinant enzyme production in isolated Bacillus subtilis 007.AMB Express. 2025 Feb 7;15(1):24. doi: 10.1186/s13568-025-01832-6. AMB Express. 2025. PMID: 39918718 Free PMC article.
References
-
- Panesar PS, Panesar R, Singh RS, Kennedy JF, Kumar H. Microbial production, immobilization and applications of β-D-galactosidase. J Chem Technol Biotechnol. 2006;81:530–43. doi: 10.1002/jctb.1453. - DOI
-
- Scotta F, Vera C, Conejeros R. Technical and economic analysis of industrial production of lactose-derived prebiotics with focus on galacto-oligosaccharides. In: Illanes A, Guerrero C, Vera C, Wilson L, Conejeros R, Scotta F, editors. Lactose-derived prebiotics: a process perspect. Elsevier Inc.; 2016. pp. 261–84. 10.1016/B978-0-12-802724-0.00007-X.
-
- Fischer L, Lutz-Wahl S. Paenibacillus wynnii beta-galactosidase for the production of lactose-depleted dairy products. EP3682748A1. 2020.
-
- Lutz-Wahl S, Mozer H, Kussler A, Schulz A, Seitl I, Fischer L. A new β-galactosidase from Paenibacillus wynnii with potential for industrial applications. J Dairy Sci. 2024;107:3429–42. 10.3168/jds.2023-24122. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

