Lysine acetyltransferase 14 mediates TGF-β-induced fibrosis in ovarian endometrioma via co-operation with serum response factor
- PMID: 38867256
- PMCID: PMC11167823
- DOI: 10.1186/s12967-024-05243-2
Lysine acetyltransferase 14 mediates TGF-β-induced fibrosis in ovarian endometrioma via co-operation with serum response factor
Abstract
Background: Fibrogenesis within ovarian endometrioma (endometrioma), mainly induced by transforming growth factor-β (TGF-β), is characterized by myofibroblast over-activation and excessive extracellular matrix (ECM) deposition, contributing to endometrioma-associated symptoms such as infertility by impairing ovarian reserve and oocyte quality. However, the precise molecular mechanisms that underpin the endometrioma- associated fibrosis progression induced by TGF-β remain poorly understood.
Methods: The expression level of lysine acetyltransferase 14 (KAT14) was validated in endometrium biopsies from patients with endometrioma and healthy controls, and the transcription level of KAT14 was further confirmed by analyzing a published single-cell transcriptome (scRNA-seq) dataset of endometriosis. We used overexpression, knockout, and knockdown approaches in immortalized human endometrial stromal cells (HESCs) or human primary ectopic endometrial stromal cells (EcESCs) to determine the role of KAT14 in TGF-β-induced fibrosis. Furthermore, an adeno-associated virus (AAV) carrying KAT14-shRNA was used in an endometriosis mice model to assess the role of KAT14 in vivo.
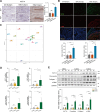
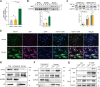
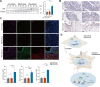
Results: KAT14 was upregulated in ectopic lesions from endometrioma patients and predominantly expressed in activated fibroblasts. In vitro studies showed that KAT14 overexpression significantly promoted a TGF-β-induced profibrotic response in endometrial stromal cells, while KAT14 silencing showed adverse effects that could be rescued by KAT14 re-enhancement. In vivo, Kat14 knockdown ameliorated fibrosis in the ectopic lesions of the endometriosis mouse model. Mechanistically, we showed that KAT14 directly interacted with serum response factor (SRF) to promote the expression of α-smooth muscle actin (α-SMA) by increasing histone H4 acetylation at promoter regions; this is necessary for TGF-β-induced ECM production and myofibroblast differentiation. In addition, the knockdown or pharmacological inhibition of SRF significantly attenuated KAT14-mediating profibrotic effects under TGF-β treatment. Notably, the KAT14/SRF complex was abundant in endometrioma samples and positively correlated with α-SMA expression, further supporting the key role of KAT14/SRF complex in the progression of endometrioma-associated fibrogenesis.
Conclusion: Our results shed light on KAT14 as a key effector of TGF-β-induced ECM production and myofibroblast differentiation in EcESCs by promoting histone H4 acetylation via co-operating with SRF, representing a potential therapeutic target for endometrioma-associated fibrosis.
Keywords: Endometrioma-associated fibrosis; Epigenetic modification; KAT14; SRF; TGF-β.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Endometriotic mesenchymal stem cells significantly promote fibrogenesis in ovarian endometrioma through the Wnt/β-catenin pathway by paracrine production of TGF-β1 and Wnt1.Hum Reprod. 2016 Jun;31(6):1224-35. doi: 10.1093/humrep/dew058. Epub 2016 Mar 22. Hum Reprod. 2016. PMID: 27005891
-
Corroborating evidence for platelet-induced epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis.Hum Reprod. 2016 Apr;31(4):734-49. doi: 10.1093/humrep/dew018. Epub 2016 Feb 22. Hum Reprod. 2016. PMID: 26908845
-
IL-10 is not anti-fibrotic but pro-fibrotic in endometriosis: IL-10 treatment of endometriotic stromal cells in vitro promotes myofibroblast proliferation and collagen type I protein expression.Hum Reprod. 2023 Jan 5;38(1):14-29. doi: 10.1093/humrep/deac248. Hum Reprod. 2023. PMID: 36413036
-
The role of TGF-β in the pathophysiology of peritoneal endometriosis.Hum Reprod Update. 2017 Sep 1;23(5):548-559. doi: 10.1093/humupd/dmx016. Hum Reprod Update. 2017. PMID: 28903471 Review.
-
The role of fibrosis in endometriosis: a systematic review.Hum Reprod Update. 2024 Dec 1;30(6):706-750. doi: 10.1093/humupd/dmae023. Hum Reprod Update. 2024. PMID: 39067455 Free PMC article.
Cited by
-
Integrated analysis of single-cell and bulk transcriptomic data reveals altered cellular composition and predictive cell types in ectopic endometriosis.Front Med (Lausanne). 2025 Jul 18;12:1641982. doi: 10.3389/fmed.2025.1641982. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40757199 Free PMC article.
-
LncPrep + 96kb inhibits ovarian fibrosis by upregulating prolyl oligopeptidase expression.Mol Med Rep. 2025 May;31(5):113. doi: 10.3892/mmr.2025.13478. Epub 2025 Feb 28. Mol Med Rep. 2025. PMID: 40017148 Free PMC article.
References
-
- Sanchez AM, Viganò P, Somigliana E, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update. 2014;20(2):217–30. doi: 10.1093/humupd/dmt053. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

