Cryo-EM structures reveal tau filaments from Down syndrome adopt Alzheimer's disease fold
- PMID: 38867338
- PMCID: PMC11167798
- DOI: 10.1186/s40478-024-01806-y
Cryo-EM structures reveal tau filaments from Down syndrome adopt Alzheimer's disease fold
Abstract
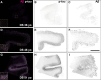
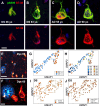
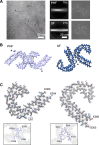
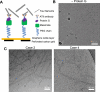
Down syndrome (DS) is a common genetic condition caused by trisomy of chromosome 21. Among their complex clinical features, including musculoskeletal, neurological, and cardiovascular disabilities, individuals with DS have an increased risk of developing progressive dementia and early-onset Alzheimer's disease (AD). This dementia is attributed to the increased gene dosage of the amyloid-β (Aβ) precursor protein gene, the formation of self-propagating Aβ and tau prion conformers, and the deposition of neurotoxic Aβ plaques and tau neurofibrillary tangles. Tau amyloid fibrils have previously been established to adopt many distinct conformations across different neurodegenerative conditions. Here, we report the characterization of brain samples from four DS cases spanning 36-63 years of age by spectral confocal imaging with conformation-specific dyes and cryo-electron microscopy (cryo-EM) to determine structures of isolated tau fibrils. High-resolution structures revealed paired helical filament (PHF) and straight filament (SF) conformations of tau that were identical to those determined from AD cases. The PHFs and SFs are made of two C-shaped protofilaments, each containing a cross-β/β-helix motif. Similar to filaments from AD cases, most filaments from the DS cases adopted the PHF form, while a minority (approximately 20%) formed SFs. Samples from the youngest individual with no documented dementia had sparse tau deposits. To isolate tau for cryo-EM from this challenging sample we used a novel affinity-grid method involving a graphene oxide surface derivatized with anti-tau antibodies. This method improved isolation and revealed that primarily tau PHFs and a minor population of chronic traumatic encephalopathy type II-like filaments were present in this youngest case. These findings expand the similarities between AD and DS to the molecular level, providing insight into their related pathologies and the potential for targeting common tau filament folds by small-molecule therapeutics and diagnostics.
Keywords: Alzheimer’s disease; Cryo–electron microscopy; Down syndrome; Protein conformation; Tauopathy.
© 2024. The Author(s).
Conflict of interest statement
S.B.P. is the founder of Prio-Pharma, which did not contribute financial or any other support to these studies. All other authors declare that they have no competing interests.
Figures

Update of
-
Cryo-EM Structures Reveal Tau Filaments from Down Syndrome Adopt Alzheimer's Disease Fold.bioRxiv [Preprint]. 2024 May 28:2024.04.02.587507. doi: 10.1101/2024.04.02.587507. bioRxiv. 2024. Update in: Acta Neuropathol Commun. 2024 Jun 12;12(1):94. doi: 10.1186/s40478-024-01806-y. PMID: 38617229 Free PMC article. Updated. Preprint.
References
-
- Vos T, Allen C, Arora M. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. - DOI - PMC - PubMed

