Growth hormone-releasing hormone deficiency confers extended lifespan and metabolic resilience during high-fat feeding in mid and late life
- PMID: 38867381
- PMCID: PMC11488314
- DOI: 10.1111/acel.14238
Growth hormone-releasing hormone deficiency confers extended lifespan and metabolic resilience during high-fat feeding in mid and late life
Abstract
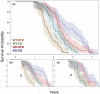
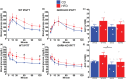
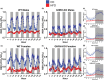
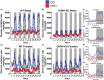
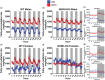
Growth hormone-releasing hormone-deficient (GHRH-KO) mice have previously been characterized by lower body weight, disproportionately high body fat accumulation, preferential metabolism of lipids compared to carbohydrates, improved insulin sensitivity, and an extended lifespan. That these mice are long-lived and insulin-sensitive conflicts with the notion that adipose tissue accumulation drives the health detriments associated with obesity (i.e., diabetes), and indicates that GH signaling may be necessary for the development of adverse effects linked to obesity. This prompts investigation into the ultimate effect of diet-induced obesity on the lifespan of these long-lived mice. To this end, we initiated high-fat feeding in mid and late-life in GHRH-KO and wild-type (WT) mice. We carried out extensive lifespan analysis coupled with glucose/insulin tolerance testing and indirect calorimetry to gauge the metabolic effect of high-fat dietary stress through adulthood on these mice. We show that under high-fat diet (HFD) conditions, GHRH-KO mice display extended lifespans relative to WT controls. We also show that GHRH-KO mice are more insulin-sensitive and display less dramatic changes in their metabolism relative to WT mice, with GHRH-KO mice fed HFD displaying respiratory exchange ratios and glucose oxidation rates comparable to control-diet fed GHRH-KO mice, while WT mice fed HFD showed significant reductions in these parameters. Our results indicate that GH deficiency protects against the adverse effects of diet-induced obesity in later life.
Keywords: aging; growth hormone‐releasing hormone; high fat diet; insulin; longevity.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
All contributing authors declare no conflict of interest.
Figures

References
-
- Ahmed, B. , Sultana, R. , & Greene, M. W. (2021). Adipose tissue and insulin resistance in obese. Biomedicine & Pharmacotherapy, 137, 111315. - PubMed
-
- Andrikopoulos, S. , Blair, A. R. , Deluca, N. , Fam, B. C. , & Proietto, J. (2008). Evaluating the glucose tolerance test in mice. American Journal of Physiology. Endocrinology and Metabolism, 295(6), E1323–E1332. - PubMed
-
- Ayala, J. E. , Bracy, D. P. , McGuinness, O. P. , & Wasserman, D. H. (2006). Considerations in the design of hyperinsulinemic‐euglycemic clamps in the conscious mouse. Diabetes, 55(2), 390–397. - PubMed
-
- Baquedano, E. , Ruiz‐Lopez, A. M. , Sustarsic, E. G. , Herpy, J. , List, E. O. , Chowen, J. A. , Frago, L. M. , Kopchick, J. J. , & Argente, J. (2014). The absence of GH signaling affects the susceptibility to high‐fat diet‐induced hypothalamic inflammation in male mice. Endocrinology, 155(12), 4856–4867. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

