Peripheral cytokine interleukin-10 alleviates perihematomal edema after intracerebral hemorrhage via interleukin-10 receptor/JAK1/STAT3 signaling
- PMID: 38867395
- PMCID: PMC11168964
- DOI: 10.1111/cns.14796
Peripheral cytokine interleukin-10 alleviates perihematomal edema after intracerebral hemorrhage via interleukin-10 receptor/JAK1/STAT3 signaling
Abstract
Aims: The extent of perihematomal edema following intracerebral hemorrhage (ICH) significantly impacts patient prognosis, and disruption of the blood-brain barrier (BBB) exacerbates perihematomal edema. However, the role of peripheral IL-10 in mitigating BBB disruption through pathways that link peripheral and central nervous system signals remains poorly understood.
Methods: Recombinant IL-10 was administered to ICH model mice via caudal vein injection, an IL-10-inhibiting adeno-associated virus and an IL-10 receptor knockout plasmid were delivered intraventricularly, and neurobehavioral deficits, perihematomal edema, BBB disruption, and the expression of JAK1 and STAT3 were evaluated.
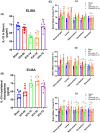
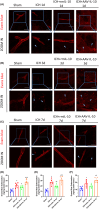
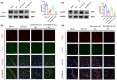
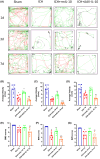
Results: Our study demonstrated that the peripheral cytokine IL-10 mitigated BBB breakdown, perihematomal edema, and neurobehavioral deficits after ICH and that IL-10 deficiency reversed these effects, likely through the IL-10R/JAK1/STAT3 signaling pathway.
Conclusions: Peripheral IL-10 has the potential to reduce BBB damage and perihematomal edema following ICH and improve patient prognosis.
Keywords: Interleukin‐10; blood–brain barrier; intracerebral hemorrhage; perihematomal edema; tight junction.
© 2024 The Authors. CNS Neuroscience & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

