Integration of Sensor-Based and Self-Reported Metrics in a Sleep Diary: A Pilot Exploration
- PMID: 38867429
- PMCID: PMC11365783
- DOI: 10.1080/15402002.2024.2359413
Integration of Sensor-Based and Self-Reported Metrics in a Sleep Diary: A Pilot Exploration
Abstract
Objectives: Discrepancies between sleep diaries and sensor-based sleep parameters are widely recognized. This study examined the effect of showing sensor-based sleep parameters while completing a daily diary. The provision of sensor-based data was expected to reduce variance but not change the mean of self-reported sleep parameters, which would in turn align better with sensor-based data compared to a control diary.
Method: In a crossover study, 24 volunteers completed week-long periods of control diary (digital sleep diary without sensor-based data feedback) or integrated diary (diary with device feedback), washout, and then the other diary condition.
Results: The integrated diary reduced self-reported total sleep time (TST) by <10 minutes and reduced variance in TST. The integrated diary did not impact mean sleep onset latency (SOL) and, unexpectedly, the variance in SOL increased. The integrated diary improved both bias and limits of agreement for SOL and TST.
Conclusions: Integration of wearable, sensor-based device data in a sleep diary has little impact on means, mixed evidence for less variance, and better agreement with sensor-based data than a traditional diary. How the diary impacts reporting and sensor-based sleep measurements should be explored.
Conflict of interest statement
Disclosure statement: Justin Brooks, MD, Ph.D., holds a provisional U.S. Patent, “Method and system to improve the accuracy of patient-reported outcomes in clinical trials” that protects the diary method described in this manuscript.
Figures


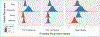



References
-
- Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, Amtmann D, Bode R, Buysse D, Choi S, Cook K, DeVellis R, DeWalt D, Fries JF, Gershon R, Hahn EA, Lai J-S, Pilkonis P, Revicki D, … Hays R (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63(11), 1179–1194. 10.1016/j.jclinepi.2010.04.011 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
