Transient pores in hemifusion diaphragms
- PMID: 38867448
- PMCID: PMC11365115
- DOI: 10.1016/j.bpj.2024.06.009
Transient pores in hemifusion diaphragms
Abstract
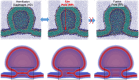
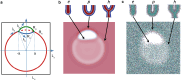
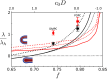
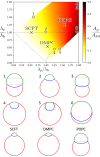
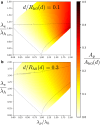
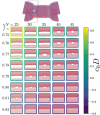
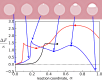
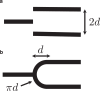
Exchange of material across two membranes, as in the case of synaptic neurotransmitter release from a vesicle, involves the formation and poration of a hemifusion diaphragm (HD). The nontrivial geometry of the HD leads to environment-dependent control, regarding the stability and dynamics of the pores required for this kind of exocytosis. This work combines particle simulations, field-based calculations, and phenomenological modeling to explore the factors influencing the stability, dynamics, and possible control mechanisms of pores in HDs. We find that pores preferentially form at the HD rim, and that their stability is sensitive to a number of factors, including the three line tensions, membrane tension, HD size, and the ability of lipids to "flip-flop" across leaflets. Along with a detailed analysis of these factors, we discuss ways that vesicles or cells may use them to open and close pores and thereby quickly and efficiently transport material.
Copyright © 2024 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Rizzoli S.O., Jahn R. Kiss-and-run, Collapse and ‘Readily Retrievable’ Vesicles. Traffic. 2007;8:1137–1144. - PubMed
-
- Haucke V., Neher E., Sigrist S.J. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 2011;12:127–138. - PubMed
-
- Kononenko N.L., Haucke V. Molecular mechanisms of presynaptic membrane retrieval and synaptic vesicle reformation. Neuron. 2015;85:484–496. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

