Nicotine-induced Genetic and Epigenetic Modifications in Primary Human Amniotic Fluid Stem Cells
- PMID: 38867535
- PMCID: PMC11348467
- DOI: 10.2174/0113816128305232240607084420
Nicotine-induced Genetic and Epigenetic Modifications in Primary Human Amniotic Fluid Stem Cells
Abstract
Background: Smoking during pregnancy has been linked to adverse health outcomes in offspring, but the underlying mechanisms are not fully understood. To date, the effect of maternal smoking has been tested in primary tissues and animal models, but the scarcity of human tissues limits experimental studies. Evidence regarding smoking-related molecular alteration and gene expression profiles in stem cells is still lacking.
Methods: We developed a cell culture model of human amniotic fluid stem cells (hAFSCs) of nicotine (NIC) exposure to examine the impact of maternal smoking on epigenetic alterations of the fetus.
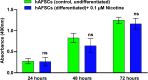
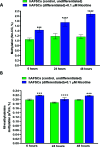
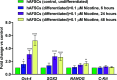
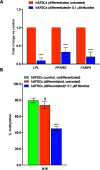
Results: NIC 0.1 μM (equivalent to "light" smoking, i.e., 5 cigarettes/day) did not significantly affect cell viability; however, significant alterations in DNA methylation and N6-methyladenosine (m6A) RNA methylation in hAFSCs occurred. These epigenetic changes may influence the gene expression and function of hAFSCs. Furthermore, NIC exposure caused time-dependent alterations of the expression of pluripotency genes and cell surface markers, suggesting enhanced cell stemness and impaired differentiation potential. Furthermore, NICtreated cells showed reduced mRNA levels of key adipogenic markers and hypomethylation of the promoter region of the imprinted gene H19 during adipogenic differentiation, potentially suppressing adipo/lipogenesis. Differential expression of 16 miRNAs, with predicted target genes involved in various metabolic pathways and linked to pathological conditions, including cognitive delay and fetal growth retardation, has been detected.
Conclusion: Our findings highlight multi-level effects of NIC on hAFSCs, including epigenetic modifications, altered gene expression, and impaired cellular differentiation, which may contribute to long-term consequences of smoking in pregnancy and its potential impact on offspring health and development.
Keywords: Primary human amniotic fluid stem cells; adipogenesis; epigenetics; mRNAs.; nicotine; tobacco-related disorders.
Conflict of interest statement
The authors declare no conflict of interest, financial or otherwise.
Figures

Similar articles
-
Genetic and epigenetic modifications induced by chemotherapeutic drugs: human amniotic fluid stem cells as an in-vitro model.BMC Med Genomics. 2019 Oct 28;12(1):146. doi: 10.1186/s12920-019-0595-3. BMC Med Genomics. 2019. PMID: 31660974 Free PMC article.
-
Urothelial differentiation of human amniotic fluid stem cells by urothelium specific conditioned medium.Cell Biol Int. 2014 Apr;38(4):531-7. doi: 10.1002/cbin.10232. Epub 2014 Jan 13. Cell Biol Int. 2014. PMID: 24375948 Free PMC article.
-
Epigenetic regulation of amniotic fluid mesenchymal stem cell differentiation to the mesodermal lineages at normal and fetus-diseased gestation.J Cell Biochem. 2020 Feb;121(2):1811-1822. doi: 10.1002/jcb.29416. Epub 2019 Oct 21. J Cell Biochem. 2020. PMID: 31633234
-
DNA methylation alterations in response to prenatal exposure of maternal cigarette smoking: A persistent epigenetic impact on health from maternal lifestyle?Arch Toxicol. 2016 Feb;90(2):231-45. doi: 10.1007/s00204-014-1426-0. Epub 2014 Dec 6. Arch Toxicol. 2016. PMID: 25480659 Review.
-
Epigenetic toxicity of heavy metals - implications for embryonic stem cells.Environ Int. 2024 Nov;193:109084. doi: 10.1016/j.envint.2024.109084. Epub 2024 Oct 18. Environ Int. 2024. PMID: 39437622 Review.
Cited by
-
Smoke signals in the genome: Epigenetic consequences of parental tobacco exposure (Review).Biomed Rep. 2025 Jun 23;23(3):146. doi: 10.3892/br.2025.2024. eCollection 2025 Sep. Biomed Rep. 2025. PMID: 40599616 Free PMC article. Review.
References
-
- Storr C.L., Cheng H., Alonso J., Angermeyer M., Bruffaerts R., de Girolamo G., de Graaf R., Gureje O., Karam E.G., Kostyuchenko S., Lee S., Lepine J.P., Medina Mora M.E., Myer L., Neumark Y., Posada-Villa J., Watanabe M., Wells J.E., Kessler R.C., Anthony J.C. Smoking estimates from around the world: Data from the first 17 participating countries in the World Mental Health Survey Consortium. Tob. Control. 2010;19(1):65–74. doi: 10.1136/tc.2009.032474. - DOI - PMC - PubMed
-
- Drake P., Driscoll A.K., Mathews T.J. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief. 2018;(305):1–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

