Upregulation of HOXA3 by isoform-specific Wilms tumour 1 drives chemotherapy resistance in acute myeloid leukaemia
- PMID: 38867543
- PMCID: PMC11448753
- DOI: 10.1111/bjh.19563
Upregulation of HOXA3 by isoform-specific Wilms tumour 1 drives chemotherapy resistance in acute myeloid leukaemia
Abstract
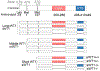
Upregulation of the Wilms' tumour 1 (WT1) gene is common in acute myeloid leukaemia (AML) and is associated with poor prognosis. WT1 generates 12 primary transcripts through different translation initiation sites and alternative splicing. The short WT1 transcripts express abundantly in primary leukaemia samples. We observed that overexpression of short WT1 transcripts lacking exon 5 with and without the KTS motif (sWT1+/- and sWT1-/-) led to reduced cell growth. However, only sWT1+/- overexpression resulted in decreased CD71 expression, G1 arrest, and cytarabine resistance. Primary AML patient cells with low CD71 expression exhibit resistance to cytarabine, suggesting that CD71 may serve as a potential biomarker for chemotherapy. RNAseq differential expressed gene analysis identified two transcription factors, HOXA3 and GATA2, that are specifically upregulated in sWT1+/- cells, whereas CDKN1A is upregulated in sWT1-/- cells. Overexpression of either HOXA3 or GATA2 reproduced the effects of sWT1+/-, including decreased cell growth, G1 arrest, reduced CD71 expression and cytarabine resistance. HOXA3 expression correlates with chemotherapy response and overall survival in NPM1 mutation-negative leukaemia specimens. Overexpression of HOXA3 leads to drug resistance against a broad spectrum of chemotherapeutic agents. Our results suggest that WT1 regulates cell proliferation and drug sensitivity in an isoform-specific manner.
Keywords: HOXA3; WT1; biomarkers; chemotherapy resistance.
© 2024 British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
No competing interests to disclose.
Figures






Similar articles
-
GATA2 links stemness to chemotherapy resistance in acute myeloid leukemia.Blood. 2025 May 8;145(19):2179-2195. doi: 10.1182/blood.2024025761. Blood. 2025. PMID: 39841459
-
Gene Expression Insights into Cytarabine Resistance in Acute Myeloid Leukemia: The Role of Cytokines.Cancer Genomics Proteomics. 2025 Sep-Oct;22(5):738-749. doi: 10.21873/cgp.20533. Cancer Genomics Proteomics. 2025. PMID: 40883022
-
Pretreatment expression of miR-191a may predict response to the induction chemotherapy based on cytarabine in acute myeloid leukemia patients - a single-center pilotal study.PLoS One. 2025 Jun 24;20(6):e0324320. doi: 10.1371/journal.pone.0324320. eCollection 2025. PLoS One. 2025. PMID: 40554554 Free PMC article.
-
A systematic overview of chemotherapy effects in acute myeloid leukaemia.Acta Oncol. 2001;40(2-3):231-52. doi: 10.1080/02841860151116321. Acta Oncol. 2001. PMID: 11441935
-
Therapies for acute myeloid leukemia in patients ineligible for standard induction chemotherapy: a systematic review.Future Oncol. 2023 Apr;19(11):789-810. doi: 10.2217/fon-2022-1286. Epub 2023 May 12. Future Oncol. 2023. PMID: 37170899
References
-
- Wang ZY, Qiu QQ, Deuel TF. The Wilms’ tumor gene product WT1 activates or suppresses transcription through separate functional domains. Journal of Biological Chemistry. 1993;268(13):. - PubMed
-
- Hastie ND. Wilms’ tumour 1 (WT1) in development, homeostasis and disease. Development (Cambridge). 2017;144(16):. - PubMed
-
- Mrowka C, Schedl A. Wilms’ tumor suppressor gene WT1: From structure to renal pathophysiologic features. Journal of the American Society of Nephrology. 2000;11(SUPPL. 16): - PubMed
MeSH terms
Substances
Grants and funding
- The Medical Research Foundation of Oregon (MRF)
- R00 CA237630/CA/NCI NIH HHS/United States
- 5K99CA237630/5 R00 CA237630-05/CA/NCI NIH HHS/United States
- K99 CA237630/CA/NCI NIH HHS/United States
- : TOP-2023-002/The Knight Cancer Institute NCI Cancer Center Support Grant (CCSG), Translational Oncology Program Pilot Award
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

