Acute exposure to dihydroxyacetone promotes genotoxicity and chromosomal instability in lung, cardiac, and liver cell models
- PMID: 38867704
- PMCID: PMC11347775
- DOI: 10.1093/toxsci/kfae075
Acute exposure to dihydroxyacetone promotes genotoxicity and chromosomal instability in lung, cardiac, and liver cell models
Abstract
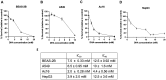
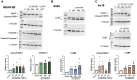
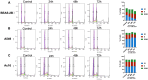
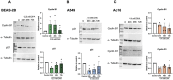
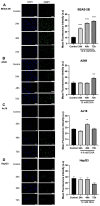
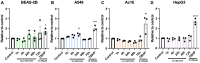
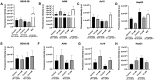
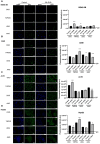
Inhalation exposures to dihydroxyacetone (DHA) occur through spray tanning and e-cigarette aerosols. Several studies in skin models have demonstrated that millimolar doses of DHA are cytotoxic, yet the genotoxicity was unclear. We examined the genotoxicity of DHA in cell models relevant to inhalation exposures. Human bronchial epithelial cells BEAS-2B, lung carcinoma cells A549, cardiomyocyte Ac16, and hepatocellular carcinoma HepG3 were exposed to DHA, and low millimolar doses of DHA were cytotoxic. IC90 DHA doses induced cell cycle arrest in all cells except the Ac16. We examined DHA's genotoxicity using strand break markers, DNA adduct detection by Repair Assisted Damage Detection (RADD), metaphase spreads, and a forward mutation assay for mutagenesis. Similar to results for skin, DHA did not induce significant levels of strand breaks. However, RADD revealed DNA adducts were induced 24 h after DHA exposure, with BEAS-2B and Ac16 showing oxidative lesions and A549 and HepG3 showing crosslink-type lesions. Yet, only low levels of reactive oxygen species or advanced glycation end products were detected after DHA exposure. Metaphase spreads revealed significant increases in chromosomal aberrations in the BEAS-2B and HepG3 with corresponding changes in ploidy. Finally, we confirmed the mutagenesis observed using the supF reporter plasmid. DHA increased the mutation frequency, consistent with methylmethane sulfonate, a mutagen and clastogen. These data demonstrate DHA is a clastogen, inducing cell-specific genotoxicity and chromosomal instability. The specific genotoxicity measured in the BEAS-2B in this study suggests that inhalation exposures pose health risks to vapers, requiring further investigation.
Keywords: DNA adducts; cytotoxicity; dihydroxyacetone; e-cigarettes; genotoxicity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society of Toxicology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures



Similar articles
-
Dihydroxyacetone Exposure Alters NAD(P)H and Induces Mitochondrial Stress and Autophagy in HEK293T Cells.Chem Res Toxicol. 2019 Aug 19;32(8):1722-1731. doi: 10.1021/acs.chemrestox.9b00230. Epub 2019 Aug 2. Chem Res Toxicol. 2019. PMID: 31328504 Free PMC article.
-
Cytotoxic, genotoxic, and toxicogenomic effects of dihydroxyacetone in human primary keratinocytes.Cutan Ocul Toxicol. 2021 Sep;40(3):232-240. doi: 10.1080/15569527.2021.1931877. Epub 2021 Jun 11. Cutan Ocul Toxicol. 2021. PMID: 34008457
-
Genotoxicity of short single-wall and multi-wall carbon nanotubes in human bronchial epithelial and mesothelial cells in vitro.Toxicology. 2013 Nov 8;313(1):24-37. doi: 10.1016/j.tox.2012.12.008. Epub 2012 Dec 21. Toxicology. 2013. PMID: 23266321
-
Exogenous exposure to dihydroxyacetone mimics high fructose induced oxidative stress and mitochondrial dysfunction.Environ Mol Mutagen. 2021 Mar;62(3):185-202. doi: 10.1002/em.22425. Epub 2021 Feb 6. Environ Mol Mutagen. 2021. PMID: 33496975 Free PMC article. Review.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
Cited by
-
Alternative Splicing of the Last TKFC Intron Yields Transcripts Differentially Expressed in Human Tissues That Code In Vitro for a Protein Devoid of Triokinase and FMN Cyclase Activity.Biomolecules. 2024 Oct 12;14(10):1288. doi: 10.3390/biom14101288. Biomolecules. 2024. PMID: 39456221 Free PMC article.
-
Inhalation exposure to dihydroxyacetone promotes lung injury and pulmonary fibrosis in A/J mice.Toxicol Rep. 2024 Dec 18;14:101878. doi: 10.1016/j.toxrep.2024.101878. eCollection 2025 Jun. Toxicol Rep. 2024. PMID: 40612655 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

