The Tricarboxylic Acid Cycle Metabolites for Cancer: Friend or Enemy
- PMID: 38867720
- PMCID: PMC11168306
- DOI: 10.34133/research.0351
The Tricarboxylic Acid Cycle Metabolites for Cancer: Friend or Enemy
Abstract
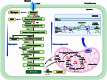
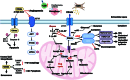
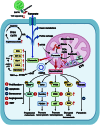
The tricarboxylic acid (TCA) cycle is capable of providing sufficient energy for the physiological activities under aerobic conditions. Although tumor metabolic reprogramming places aerobic glycolysis in a dominant position, the TCA cycle remains indispensable for tumor cells as a hub for the metabolic linkage and interconversion of glucose, lipids, and certain amino acids. TCA intermediates such as citrate, α-ketoglutarate, succinate, and fumarate are altered in tumors, and they regulate the tumor metabolism, signal transduction, and immune environment to affect tumorigenesis and tumor progression. This article provides a comprehensive review of the modifications occurring in tumor cells in relation to the intermediates of the TCA cycle, which affects tumor pathogenesis and current therapeutic strategy for therapy through targeting TCA cycle in cancer cells.
Copyright © 2024 Jie Wu et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


References
-
- Inigo M, Deja S, Burgess SC. Ins and outs of the TCA cycle: The central role of anaplerosis. Annu Rev Nutr. 2021;41:19–47. - PubMed
-
- Greene J, Segaran A, Lord S. Targeting OXPHOS and the electron transport chain in cancer; molecular and therapeutic implications. Semin Cancer Biol. 2022;86(Pt 2):851–859. - PubMed
-
- Icard P, Shulman S, Farhat D, Steyaert JM, Alifano M, Lincet H. How the Warburg effect supports aggressiveness and drug resistance of cancer cells? Drug Resist Updat. 2018;38:1–11. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

