Synthesis and medicinal chemical characterisation of antiproliferative O, N-functionalised isopulegol derivatives
- PMID: 38867736
- PMCID: PMC11168086
- DOI: 10.1039/d4ra03467h
Synthesis and medicinal chemical characterisation of antiproliferative O, N-functionalised isopulegol derivatives
Abstract
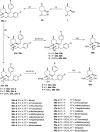
Benzylation of isopulegol furnished O-benzyl-protected isopulegol, which was transformed into aminodiols via epoxidation followed by ring opening of the corresponding epoxides and subsequent hydrogenolysis. On the other hand, (-)-isopulegol was oxidised to a diol, which was then converted into dibenzyl-protected diol derivatives. The products were then transformed into aminotriols by using a similar method. The antiproliferative activity of aminodiol and aminotriol derivatives was examined. In addition, structure-activity relationships were also explored from the aspects of substituent effects and stereochemistry on the aminodiol and aminotriol systems. The drug-likeness of the compounds was assessed by in silico and experimental physicochemical characterisations, completed by kinetic aqueous solubility and in vitro intestinal-specific parallel artificial membrane permeability assay (PAMPA-GI) measurements.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds.RSC Adv. 2020 Oct 19;10(63):38468-38477. doi: 10.1039/d0ra07739a. eCollection 2020 Oct 15. RSC Adv. 2020. PMID: 35517552 Free PMC article.
-
Stereoselective Synthesis and Investigation of Isopulegol-Based Chiral Ligands.Int J Mol Sci. 2019 Aug 19;20(16):4050. doi: 10.3390/ijms20164050. Int J Mol Sci. 2019. PMID: 31430981 Free PMC article.
-
Stereoselective Synthesis and Antiproliferative Activities of Tetrafunctional Diterpene Steviol Derivatives.Int J Mol Sci. 2023 Jan 6;24(2):1121. doi: 10.3390/ijms24021121. Int J Mol Sci. 2023. PMID: 36674639 Free PMC article.
-
Cephalostatin analogues--synthesis and biological activity.Fortschr Chem Org Naturst. 2004;87:1-80. doi: 10.1007/978-3-7091-0581-8_1. Fortschr Chem Org Naturst. 2004. PMID: 15079895 Review.
-
Enantiomeric Isopulegol as the Chiral Pool in the Total Synthesis of Bioactive Agents.Chem Rec. 2022 Jan;22(1):e202100194. doi: 10.1002/tcr.202100194. Epub 2021 Sep 23. Chem Rec. 2022. PMID: 34553822 Review.
References
-
- González-Rosende M. E. Jordá-Gregori J. M. Sepúlveda-Arques J. Orena M. Tetrahedron: Asymmetry. 2004;15:419–422. doi: 10.1016/j.tetasy.2003.12.022. - DOI
-
- Guarnieri W. Sendzik M. Fröhlich R. Hoppe D. Synthesis. 1998:1274–1286. doi: 10.1055/s-1998-6099. - DOI
-
- Kamimura A. Yoshihara K. Marumo S. Yamamoto A. Nishiguchi T. Kakehi A. Hori K. J. Org. Chem. 1992;57:5403–5413. doi: 10.1021/jo00046a023. - DOI
LinkOut - more resources
Full Text Sources

