Dual excitation spectral autofluorescence lifetime and reflectance imaging for fast macroscopic characterization of tissues
- PMID: 38867800
- PMCID: PMC11166421
- DOI: 10.1364/BOE.505220
Dual excitation spectral autofluorescence lifetime and reflectance imaging for fast macroscopic characterization of tissues
Abstract
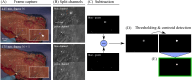
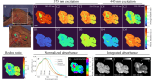
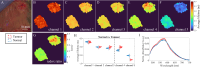
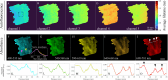
Advancements in optical imaging techniques have revolutionized the field of biomedical research, allowing for the comprehensive characterization of tissues and their underlying biological processes. Yet, there is still a lack of tools to provide quantitative and objective characterization of tissues that can aid clinical assessment in vivo to enhance diagnostic and therapeutic interventions. Here, we present a clinically viable fiber-based imaging system combining time-resolved spectrofluorimetry and reflectance spectroscopy to achieve fast multiparametric macroscopic characterization of tissues. An essential feature of the setup is its ability to perform dual wavelength excitation in combination with recording time-resolved fluorescence data in several spectral intervals. Initial validation of this bimodal system was carried out in freshly resected human colorectal cancer specimens, where we demonstrated the ability of the system to differentiate normal from malignant tissues based on their autofluorescence and reflectance properties. To further highlight the complementarity of autofluorescence and reflectance measurements and demonstrate viability in a clinically relevant scenario, we also collected in vivo data from the skin of a volunteer. Altogether, integration of these modalities in a single platform can offer multidimensional characterization of tissues, thus facilitating a deeper understanding of biological processes and potentially advancing diagnostic and therapeutic approaches in various medical applications.
© 2024 Optica Publishing Group.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Skala M. C., Riching K. M., Gendron-Fitzpatrick A., et al. , “In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia,” Proc. Natl. Acad. Sci. U.S.A. 104(49), 19494–19499 (2007). 10.1073/pnas.0708425104 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
