Bone-targeted lipoplex-loaded three-dimensional bioprinting bilayer scaffold enhanced bone regeneration
- PMID: 38867890
- PMCID: PMC11167398
- DOI: 10.1093/rb/rbae055
Bone-targeted lipoplex-loaded three-dimensional bioprinting bilayer scaffold enhanced bone regeneration
Abstract
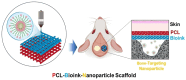
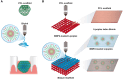
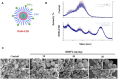
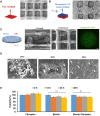
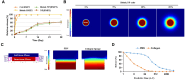
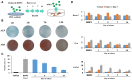
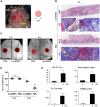
Clinical bone-morphogenetic protein 2 (BMP2) treatment for bone regeneration, often resulting in complications like soft tissue inflammation and ectopic ossification due to high dosages and non-specific delivery systems, necessitates research into improved biomaterials for better BMP2 stability and retention. To tackle this challenge, we introduced a groundbreaking bone-targeted, lipoplex-loaded, three-dimensional bioprinted bilayer scaffold, termed the polycaprolactone-bioink-nanoparticle (PBN) scaffold, aimed at boosting bone regeneration. We encapsulated BMP2 within the fibroin nanoparticle based lipoplex (Fibroplex) and functionalized it with DSS6 for bone tissue-specific targeting. 3D printing technology enables customized, porous PCL scaffolds for bone healing and soft tissue growth, with a two-step bioprinting process creating a cellular lattice structure and a bioink grid using gelatin-alginate hydrogel and DSS6-Fibroplex, shown to support effective nutrient exchange and cell growth at specific pore sizes. The PBN scaffold is predicted through in silico analysis to exhibit biased BMP2 release between bone and soft tissue, a finding validated by in vitro osteogenic differentiation assays. The PBN scaffold was evaluated for critical calvarial defects, focusing on sustained BMP2 delivery, prevention of soft tissue cell infiltration and controlled fiber membrane pore size in vivo. The PBN scaffold demonstrated a more than eight times longer BMP2 release time than that of the collagen sponge, promoting osteogenic differentiation and bone regeneration in a calvarial defect animal. Our findings suggest that the PBN scaffold enhanced the local concentration of BMP2 in bone defects through sustained release and improved the spatial arrangement of bone formation, thereby reducing the risk of heterotopic ossification.
Keywords: 3D bioprinting; BMP2; bone regeneration; bone-targeting lipoplex.
© The Author(s) 2024. Published by Oxford University Press.
Figures
Similar articles
-
Improved bone regeneration with bone targeted scaffold.Sci Rep. 2025 Jul 24;15(1):27002. doi: 10.1038/s41598-025-12497-3. Sci Rep. 2025. PMID: 40707689 Free PMC article.
-
3D Bioprinting of a Bioactive Composite Scaffold for Cell Delivery in Periodontal Tissue Regeneration.Biomolecules. 2023 Jun 30;13(7):1062. doi: 10.3390/biom13071062. Biomolecules. 2023. PMID: 37509098 Free PMC article.
-
Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect.Acta Biomater. 2018 Sep 15;78:36-47. doi: 10.1016/j.actbio.2018.07.050. Epub 2018 Jul 29. Acta Biomater. 2018. PMID: 30067947
-
3D Bioprinted Scaffolds for Bone Tissue Engineering: State-Of-The-Art and Emerging Technologies.Front Bioeng Biotechnol. 2022 Apr 11;10:824156. doi: 10.3389/fbioe.2022.824156. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35480972 Free PMC article. Review.
-
Therapeutic Potential of Nano-Sustained-Release Factors for Bone Scaffolds.J Funct Biomater. 2025 Apr 9;16(4):136. doi: 10.3390/jfb16040136. J Funct Biomater. 2025. PMID: 40278244 Free PMC article. Review.
Cited by
-
Bone-targeting engineered milk-derived extracellular vesicles for MRI-assisted therapy of osteoporosis.Regen Biomater. 2024 Sep 13;11:rbae112. doi: 10.1093/rb/rbae112. eCollection 2024. Regen Biomater. 2024. PMID: 39323741 Free PMC article.
-
Engineering 3D-BMSC exosome-based hydrogels that collaboratively regulate bone microenvironment and promote osteogenesis for enhanced cell-free bone regeneration.Mater Today Bio. 2025 May 20;32:101881. doi: 10.1016/j.mtbio.2025.101881. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40510837 Free PMC article.
-
A Collagen Membrane Pretreated with Citrate Promotes Collagen Mineralization and Bone Regeneration.J Funct Biomater. 2025 Jul 15;16(7):261. doi: 10.3390/jfb16070261. J Funct Biomater. 2025. PMID: 40710475 Free PMC article.
-
Improved bone regeneration with bone targeted scaffold.Sci Rep. 2025 Jul 24;15(1):27002. doi: 10.1038/s41598-025-12497-3. Sci Rep. 2025. PMID: 40707689 Free PMC article.
References
-
- Sierra-García GD, Castro-Ríos R, Gónzalez-Horta A, Lara-Arias J, Chávez-Montes A.. Bone morphogenetic proteins (BMP): clinical application for reconstruction of bone defects. Gac Med Mex 2016;152:381–5. - PubMed
-
- Razzouk S, Sarkis R.. BMP-2: biological challenges to its clinical use. N Y State Dent J 2012;78:37–9. - PubMed
-
- Haft GF. Is off-label use of BMP in pediatric spine surgery now a standard of care? Commentary on an article by Amit Jain, MD, et al.: “Factors associated with use of bone morphogenetic protein during pediatric spinal fusion surgery. An analysis of 4817 patients.” J Bone Joint Surg Am 2013;95:e103 1–2. - PubMed
-
- Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG.. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976) 2006;31:2813–9. - PubMed
-
- Kawakami M, Hashizume H, Matsumoto T, Enyo Y, Okada M, Yoshida M, Chubinskaya S.. Safety of epidural administration of osteogenic protein-1 (OP-1/BMP-7): behavioral and macroscopic observation. Spine (Phila Pa 1976)) 2007;32:1388–93. - PubMed
LinkOut - more resources
Full Text Sources

