Efficacy of rituximab in treating steroid-resistant Graves' orbitopathy in active moderate-to-severe and sight-threatening forms: A retrospective observation from China
- PMID: 38867959
- PMCID: PMC11167350
- DOI: 10.1016/j.heliyon.2024.e31932
Efficacy of rituximab in treating steroid-resistant Graves' orbitopathy in active moderate-to-severe and sight-threatening forms: A retrospective observation from China
Abstract
Background and objectives: The efficacy of rituximab (RTX) in treating steroid-resistant Graves' orbitopathy (GO) has been limitedly studied in Asians. Moreover, RTX has been considered even less for patients with steroid-resistant dysthyroid optic neuropathy (DON) who failed to undergo orbital decompression surgery for physical or financial reasons, or who responded poorly to the procedure. This study aimed to investigate the efficacy of RTX in treating steroid-resistant active moderate-to-severe and sight-threatening GO in a Chinese population.
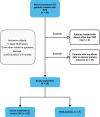
Methods: Data from 28 patients with steroid-resistant GO prescribed a single dose of 500 mg RTX were retrospectively retrieved. Treatment responses and contributing factors were analyzed.
Results: The median follow-up time was 22 (8-34) weeks. 23 (82.1 %) patients had a positive objective outcome recommended by the European Group on Graves' Orbitopathy (EUGOGO), while 25 (92.6 %) had a decrease in 7-item clinical activity score (CAS) by at least 2. Diplopia, visual dysfunction, and MRI-detected T2 relaxation time of the involved extraocular muscles improved significantly at the last follow-up compared to baseline (81.0 % vs. 47.6 %, 38.9 % vs. 16.7 %, and 87.8 (8.64) vs. 75.8 (10.9) ms, respectively; all p values < 0.05). No significant improvement was seen in terms of proptosis and eye muscle duction. Notably, a higher baseline IgG4 to IgG ratio was a predictor for RTX-induced positive EUGOGO outcomes. After RTX treatment, all 8 patients with DON demonstrated inactivation, and 4 improved in visual acuity by ≥ 1 line. No patient with DON experienced obvious deterioration.
Conclusion: A single dose of 500 mg RTX seemed to be an effective and tolerable treatment for steroid-resistant GO. However, larger-scale studies with a control group are required for a more solid conclusion. The role of RTX in steroid-resistant DON management where surgery is unavailable or ineffective should be further explored.
Keywords: Dysthyroid optic neuropathy; Rituximab; Steroid-resistant graves' orbitopathy.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Sight-threatening Graves' orbitopathy: Twenty years' experience of a multidisciplinary thyroid-eye outpatient clinic.Clin Endocrinol (Oxf). 2019 Jan;90(1):208-213. doi: 10.1111/cen.13880. Epub 2018 Nov 19. Clin Endocrinol (Oxf). 2019. PMID: 30339291
-
Therapy With Different Dose Regimens of Rituximab in Patients With Active Moderate-To-Severe Graves' Orbitopathy.Front Endocrinol (Lausanne). 2022 Jan 25;12:790246. doi: 10.3389/fendo.2021.790246. eCollection 2021. Front Endocrinol (Lausanne). 2022. PMID: 35145479 Free PMC article.
-
Optimization of the treatment of moderate to severe and active thyroid orbitopathy considering the recommendations of the European Group on Graves' Orbitopathy (EUGOGO) [Optymalizacja leczenia umiarkowanej do ciężkiej i aktywnej orbitopatii tarczycowej z uwzględnieniem zaleceń European Group on Graves' Orbitopathy (EUGOGO)].Endokrynol Pol. 2022;73(4):756-777. doi: 10.5603/EP.a2022.0040. Endokrynol Pol. 2022. PMID: 36059167 English.
-
Insights into Current Management Strategies for Dysthyroid Optic Neuropathy: A Review.Clin Ophthalmol. 2022 Mar 18;16:841-850. doi: 10.2147/OPTH.S284609. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35330749 Free PMC article. Review.
-
MANAGEMENT OF ENDOCRINE DISEASE: Rituximab therapy for Graves' orbitopathy - lessons from randomized control trials.Eur J Endocrinol. 2017 Feb;176(2):R101-R109. doi: 10.1530/EJE-16-0552. Epub 2016 Oct 19. Eur J Endocrinol. 2017. PMID: 27760790 Review.
Cited by
-
Comparative efficacy and safety of rituximab, tocilizumab, and teprotumumab in Graves' orbitopathy: a systematic review and meta-analysis.Eye (Lond). 2025 Jul;39(10):1901-1932. doi: 10.1038/s41433-025-03845-8. Epub 2025 May 22. Eye (Lond). 2025. PMID: 40404973 Review.
References
-
- Laurberg P., Berman D.C., Bülow Pedersen I., Andersen S., Carlé A. Incidence and clinical presentation of moderate to severe Graves' orbitopathy in a Danish population before and after iodine fortification of salt. J. Clin. Endocrinol. Metabol. 2012;97:2325–2332. doi: 10.1210/jc.2012-1275. - DOI - PMC - PubMed
-
- Bartalena L., Kahaly G.J., Baldeschi L., Dayan C.M., Eckstein A., Marcocci C., et al. The 2021 European Group on Graves' orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves' orbitopathy. Eur. J. Endocrinol. 2021;185:G43–G67. doi: 10.1530/EJE-21-0479. - DOI - PubMed
LinkOut - more resources
Full Text Sources