Exposing kinetic disparities between inflammasome readouts using time-resolved analysis
- PMID: 38867997
- PMCID: PMC11168392
- DOI: 10.1016/j.heliyon.2024.e32023
Exposing kinetic disparities between inflammasome readouts using time-resolved analysis
Abstract
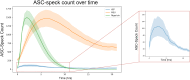
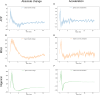
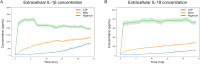
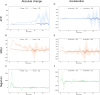
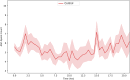
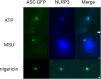
The NLRP3 inflammasome is an intracellular multiprotein complex described to be involved in both an effective host response to infectious agents and various diseases. Investigation into the NLRP3 inflammasome has been extensive in the past two decades, and often revolves around the analysis of a few specific readouts, including ASC-speck formation, caspase-1 cleavage or activation, and cleavage and release of IL-1β and/or IL-18. Quantification of these readouts is commonly undertaken as an endpoint analysis, where the presence of each positive outcome is assessed independently of the others. In this study, we apply time-resolved analysis of a human macrophage model (differentiated THP-1-ASC-GFP cells) to commonly accessible methods. This approach yields the additional quantifiable metrics time-resolved absolute change and acceleration, allowing comparisons between readouts. Using this methodological approach, we reveal (potential) discrepancies between inflammasome-related readouts that otherwise might go undiscovered. The study highlights the importance of time-resolved data in general and may be further extended as well as incorporated into other areas of research.
Keywords: ASC-Specks; Cell response; Cytokines; Human macrophages; LDH leakage; Live-cell imaging; NLRP3 inflammasome; THP-1 cells.
© 2024 The Author(s).
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:co-author affiliated to Nanoxis Consulting AB - R.K. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Soriano-Teruel P.M., García-Laínez G., Marco-Salvador M., Pardo J., Arias M., DeFord C., Merfort I., Vicent M.J., Pelegrín P., Sancho M., Orzáez M. Identification of an ASC oligomerization inhibitor for the treatment of inflammatory diseases. Cell Death & Disease. 2021;12:1155. doi: 10.1038/s41419-021-04420-1. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

