Cell Membrane Hybrid Lipid Nanovesicles Enhance Innate Immunity for Synergistic Immunotherapy by Promoting Immunogenic Cell Death and cGAS Activation
- PMID: 38868091
- PMCID: PMC11168305
- DOI: 10.34133/bmr.0038
Cell Membrane Hybrid Lipid Nanovesicles Enhance Innate Immunity for Synergistic Immunotherapy by Promoting Immunogenic Cell Death and cGAS Activation
Abstract
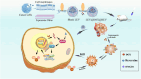
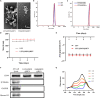
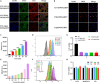
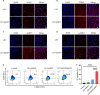
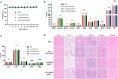
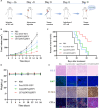
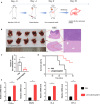
Immunotherapy shows great therapeutic potential for long-term protection against tumor relapse and metastasis. Innate immune sensors, such as cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING), dissolve DNA and induce type I interferon. Through activation of the cGAS/STING pathway, chemotherapy drugs and reversine (REV) may provide synergetic anti-tumor effects. Here, we prepared drug-loaded cell membrane hybrid lipid nanovesicles (LEVs) (designated LEV@DOX@REV) by fusion of cell membranes, phospholipids, doxorubicin (DOX), and REV, to realize accurate delivery to tumors and chemo-immunotherapy. The cell membranes of LEVs confer "homing" abilities. DOX can induce immunogenic cell death as a result of its specific immunomodulatory effects, which promotes the maturation of immune cells and improves the microenvironment of the immune system. REV is proven to efficiently activate cGAS/STING signaling, thereby enhancing the immune system. The antitumor efficacy of LEV@DOX@REV was evaluated in a 4T1 subcutaneous tumor xenograft model, a distant metastatic tumor model, and a liver metastatic tumor model. LEV@DOX@REV facilitated the infiltration of cytotoxic T lymphocytes within tumors, increased the secretion of proinflammatory cytokines, and modified the tumor microenvironment. In conclusion, LEV@DOX@REV displayed favorable antitumor effects and extended the survival of tumor-bearing mice. We therefore successfully developed nanoparticles capable of enhancing immune activation that have potential therapeutic applications for cancer immunotherapy.
Copyright © 2024 Ruijie Qian et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures
Similar articles
-
Tumor-derived nanovesicles enhance cancer synergistic chemo-immunotherapy by promoting cGAS/STING pathway activation and immunogenetic cell death.Life Sci. 2024 Jul 1;348:122687. doi: 10.1016/j.lfs.2024.122687. Epub 2024 May 7. Life Sci. 2024. PMID: 38718856
-
A homologous-targeting cGAS-STING agonist multimodally activates dendritic cells for enhanced cancer immunotherapy.Acta Biomater. 2024 Mar 15;177:400-413. doi: 10.1016/j.actbio.2024.02.003. Epub 2024 Feb 8. Acta Biomater. 2024. PMID: 38336268
-
Manganese-Based Nanoactivator Optimizes Cancer Immunotherapy via Enhancing Innate Immunity.ACS Nano. 2020 Apr 28;14(4):3927-3940. doi: 10.1021/acsnano.9b06111. Epub 2020 Apr 20. ACS Nano. 2020. PMID: 32298077
-
Emerging mechanisms and implications of cGAS-STING signaling in cancer immunotherapy strategies.Cancer Biol Med. 2024 Jan 3;21(1):45-64. doi: 10.20892/j.issn.2095-3941.2023.0440. Cancer Biol Med. 2024. PMID: 38172538 Free PMC article. Review.
-
Targeting STING for cancer immunotherapy: From mechanisms to translation.Int Immunopharmacol. 2022 Dec;113(Pt A):109304. doi: 10.1016/j.intimp.2022.109304. Epub 2022 Oct 14. Int Immunopharmacol. 2022. PMID: 36252492 Review.
Cited by
-
Leveraging nature's nanocarriers: Translating insights from extracellular vesicles to biomimetic synthetic vesicles for biomedical applications.Sci Adv. 2025 Feb 28;11(9):eads5249. doi: 10.1126/sciadv.ads5249. Epub 2025 Feb 26. Sci Adv. 2025. PMID: 40009680 Free PMC article. Review.
-
How has the field of immunogenic cell death in breast cancer evolved and impacted clinical practice over the past eleven years?Hum Vaccin Immunother. 2025 Dec;21(1):2505349. doi: 10.1080/21645515.2025.2505349. Epub 2025 May 26. Hum Vaccin Immunother. 2025. PMID: 40418649 Free PMC article. Review.
-
pH-Activated Nanoplatform Derived from M1 Macrophages' Exosomes for Photodynamic and Ferroptosis Synergistic Therapy to Augment Cancer Immunotherapy.Biomater Res. 2025 Mar 6;29:0153. doi: 10.34133/bmr.0153. eCollection 2025. Biomater Res. 2025. PMID: 40051791 Free PMC article.
-
Enhanced Cerenkov radiation induced photodynamic therapy based on GSH-responsive biomimetic nanoplatform to trigger immunogenic cell death for tumor immunotherapy.Asian J Pharm Sci. 2025 Aug;20(4):101070. doi: 10.1016/j.ajps.2025.101070. Epub 2025 May 27. Asian J Pharm Sci. 2025. PMID: 40757250 Free PMC article.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. - PubMed
-
- Ghislain I, Zikos E, Coens C, Quinten C, Balta V, Tryfonidis K, Piccart M, Zardavas D, Nagele E, Bjelic-Radisic V, et al. . Health-related quality of life in locally advanced and metastatic breast cancer: Methodological and clinical issues in randomised controlled trials. Lancet Oncol. 2016;17(7):e294–e304. - PubMed
-
- Franzoi MA, Romano E, Piccart M. Immunotherapy for early breast cancer: Too soon, too superficial, or just right? Ann Oncol. 2021;32(3):323–336. - PubMed
-
- Li Q, Liu X, Yan C, Zhao B, Zhao Y, Yang L, Shi M, Yu H, Li X, Luo K. Polysaccharide-based stimulus-responsive nanomedicines for combination cancer immunotherapy. Small. 2023;19(23):e2206211. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

