Acute onset nitrofurantoin-induced autoimmune hepatitis after urinary tract infection treatment
- PMID: 38868111
- PMCID: PMC11166554
- DOI: 10.1002/ccr3.9050
Acute onset nitrofurantoin-induced autoimmune hepatitis after urinary tract infection treatment
Abstract
Key clinical message: This case signifies the importance of recognizing DIAIH within the context of antibiotic therapy, especially in older adults and even shortly after common drug exposures for treating UTI.
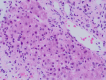
Abstract: Various drugs can induce immune-mediated liver damage and in rare instances may lead to autoimmune hepatitis. Here we report an 84-year-old woman who developed autoimmune hepatitis less than 3 weeks after treatment for urinary tract infection with the antibiotic nitrofurantoin. She presented with jaundice, right upper quadrant abdominal pain, nausea, and vomiting. In the absence of a history of an autoimmune disorder or elevated liver enzymes in the past; elevated liver enzymes after a short course of Nitrofurantoin and the presence of smooth muscle antibodies strongly suggested autoimmune hepatitis, which was confirmed through biopsy sample analysis. The patient scored 7 points on the Naranjo adverse reaction probability scale. The patient's rapid recovery within 1 month of prednisone therapy supports the association of liver damage with nitrofurantoin use.
Keywords: Naranjo adverse reaction; drug‐induced autoimmune hepatitis; elevated liver enzymes; nitrofurantoin toxicity.
© 2024 The Author(s). Clinical Case Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
[Autoimmune hepatitis triggered by nitrofurantoin: A rare drug-induced toxicity].Rev Med Interne. 2016 Feb;37(2):131-4. doi: 10.1016/j.revmed.2015.07.003. Epub 2015 Sep 26. Rev Med Interne. 2016. PMID: 26404522 French.
-
Seropositive Neuromyelitis Optica in a Case of Undiagnosed Ankylosing Spondylitis: A Neuro-Rheumatological Conundrum.Qatar Med J. 2022 Jul 7;2022(3):29. doi: 10.5339/qmj.2022.29. eCollection 2022. Qatar Med J. 2022. PMID: 35864917 Free PMC article.
-
Nitrofurantoin-induced immune-mediated lung and liver disease.Vojnosanit Pregl. 2012 Jun;69(6):536-40. Vojnosanit Pregl. 2012. PMID: 22779302
-
Drug-induced autoimmune hepatitis: A minireview.World J Gastroenterol. 2022 Jun 28;28(24):2654-2666. doi: 10.3748/wjg.v28.i24.2654. World J Gastroenterol. 2022. PMID: 35979160 Free PMC article. Review.
-
Nitrofurantoin-induced hepatotoxicity: a rare yet serious complication.South Med J. 2014 Feb;107(2):107-13. doi: 10.1097/SMJ.0000000000000059. South Med J. 2014. PMID: 24926677 Review.
Cited by
-
Drug-Induced Autoimmune Hepatitis: Robust Causality Assessment Using Two Different Validated and Scoring Diagnostic Algorithms.Diagnostics (Basel). 2025 Jun 23;15(13):1588. doi: 10.3390/diagnostics15131588. Diagnostics (Basel). 2025. PMID: 40647587 Free PMC article. Review.
References
-
- Base de dados europeia de notificações de reações adversas medicamentosas suspeitas. European Medicines Agency, Eudravigilance Publisehd in 2020. Available from: https://dap.ema.europa.eu/analytics/saw.dll?PortalPages
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous