Functional specialization of medial and lateral orbitofrontal cortex in inferential decision-making
- PMID: 38868183
- PMCID: PMC11167445
- DOI: 10.1016/j.isci.2024.110007
Functional specialization of medial and lateral orbitofrontal cortex in inferential decision-making
Abstract
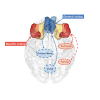
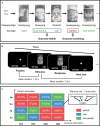
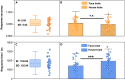
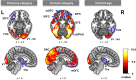
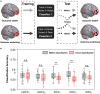
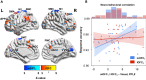
Inferring prospective outcomes and updating behavior are prerequisites for making flexible decisions in the changing world. These abilities are highly associated with the functions of the orbitofrontal cortex (OFC) in humans and animals. The functional specialization of OFC subregions in decision-making has been established in animals. However, the understanding of how human OFC contributes to decision-making remains limited. Therefore, we studied this issue by examining the information representation and functional interactions of human OFC subregions during inference-based decision-making. We found that the medial OFC (mOFC) and lateral OFC (lOFC) collectively represented the inferred outcomes which, however, were context-general coding in the mOFC and context-specific in the lOFC. Furthermore, the mOFC-motor and lOFC-frontoparietal functional connectivity may indicate the motor execution of mOFC and the cognitive control of lOFC during behavioral updating. In conclusion, our findings support the dissociable functional roles of OFC subregions in decision-making.
Keywords: Behavioral neuroscience; Neuroscience; Psychology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat.J Neurosci. 2011 Apr 27;31(17):6398-404. doi: 10.1523/JNEUROSCI.6620-10.2011. J Neurosci. 2011. PMID: 21525280 Free PMC article.
-
Contrasting Effects of Medial and Lateral Orbitofrontal Cortex Lesions on Credit Assignment and Decision-Making in Humans.J Neurosci. 2017 Jul 19;37(29):7023-7035. doi: 10.1523/JNEUROSCI.0692-17.2017. Epub 2017 Jun 19. J Neurosci. 2017. PMID: 28630257 Free PMC article.
-
The medial and lateral orbitofrontal cortex jointly represent the cognitive map of task space.Commun Biol. 2025 Feb 3;8(1):163. doi: 10.1038/s42003-025-07588-w. Commun Biol. 2025. PMID: 39900714 Free PMC article.
-
Cortico-Striatal-Thalamic Loop Circuits of the Orbitofrontal Cortex: Promising Therapeutic Targets in Psychiatric Illness.Front Syst Neurosci. 2017 Apr 27;11:25. doi: 10.3389/fnsys.2017.00025. eCollection 2017. Front Syst Neurosci. 2017. PMID: 28496402 Free PMC article. Review.
-
Amygdala-cortical collaboration in reward learning and decision making.Elife. 2022 Sep 5;11:e80926. doi: 10.7554/eLife.80926. Elife. 2022. PMID: 36062909 Free PMC article. Review.
Cited by
-
A dynamic framework of brain functional patterns shaped by spontaneous thoughts beyond the default mode network.Sci Rep. 2025 Aug 4;15(1):28389. doi: 10.1038/s41598-025-10432-0. Sci Rep. 2025. PMID: 40759678 Free PMC article.
-
White matter abnormalities of the frontal-striatal-thalamic circuit in individuals with attenuated positive symptom syndromes: a probabilistic tractography study.Schizophrenia (Heidelb). 2025 Jun 18;11(1):89. doi: 10.1038/s41537-025-00635-9. Schizophrenia (Heidelb). 2025. PMID: 40533481 Free PMC article.
-
Twin differences in the Minnesota Trust Game relate to neural mechanisms of suspiciousness.Cogn Affect Behav Neurosci. 2025 Jul 10. doi: 10.3758/s13415-025-01324-x. Online ahead of print. Cogn Affect Behav Neurosci. 2025. PMID: 40640637
-
Executive function, limbic circuit dynamics and repetitive and restricted behaviors in children with autism spectrum disorder.Front Neurosci. 2025 Jan 15;18:1508077. doi: 10.3389/fnins.2024.1508077. eCollection 2024. Front Neurosci. 2025. PMID: 39881807 Free PMC article.
References
LinkOut - more resources
Full Text Sources

