Distinct features of ribonucleotides within genomic DNA in Aicardi-Goutières syndrome ortholog mutants of Saccharomyces cerevisiae
- PMID: 38868188
- PMCID: PMC11166700
- DOI: 10.1016/j.isci.2024.110012
Distinct features of ribonucleotides within genomic DNA in Aicardi-Goutières syndrome ortholog mutants of Saccharomyces cerevisiae
Abstract
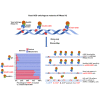
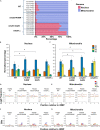
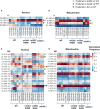
Ribonucleoside monophosphates (rNMPs) are abundantly found within genomic DNA of cells. The embedded rNMPs alter DNA properties and impact genome stability. Mutations in ribonuclease (RNase) H2, a key enzyme for rNMP removal, are associated with the Aicardi-Goutières syndrome (AGS), a severe neurological disorder. Here, we engineered orthologs of the human RNASEH2A-G37S and RNASEH2C-R69W AGS mutations in yeast Saccharomyces cerevisiae: rnh201-G42S and rnh203-K46W. Using the ribose-seq technique and the Ribose-Map bioinformatics toolkit, we unveiled rNMP abundance, composition, hotspots, and sequence context in these AGS-ortholog mutants. We found a high rNMP presence in the nuclear genome of rnh201-G42S-mutant cells, and an elevated rCMP content in both mutants, reflecting preferential cleavage of RNase H2 at rGMP. We discovered unique rNMP patterns in each mutant, showing differential activity of the AGS mutants on the leading or lagging replication strands. This study guides future research on rNMP characteristics in human genomes with AGS mutations.
Keywords: genomics; model organism; molecular genetics; nucleic acids.
© 2024 The Authors.
Conflict of interest statement
We have a patent related to this study: Storici, F., Hesselberth, J.R., and Koh, K. D. Methods to detect Ribonucleotides in deoxyribonucleic acids. GTRC-6522, 2013; U.S. 10,787,703 B1 Sep. 29, 2020. https://uspto.report/patent/grant/10,787,703.
Figures



Update of
-
Distinct features of ribonucleotides within genomic DNA in Aicardi-Goutières syndrome (AGS)-ortholog mutants of Saccharomyces cerevisiae.bioRxiv [Preprint]. 2023 Oct 2:2023.10.02.560505. doi: 10.1101/2023.10.02.560505. bioRxiv. 2023. Update in: iScience. 2024 May 16;27(6):110012. doi: 10.1016/j.isci.2024.110012. PMID: 37873120 Free PMC article. Updated. Preprint.
Similar articles
-
Distinct features of ribonucleotides within genomic DNA in Aicardi-Goutières syndrome (AGS)-ortholog mutants of Saccharomyces cerevisiae.bioRxiv [Preprint]. 2023 Oct 2:2023.10.02.560505. doi: 10.1101/2023.10.02.560505. bioRxiv. 2023. Update in: iScience. 2024 May 16;27(6):110012. doi: 10.1016/j.isci.2024.110012. PMID: 37873120 Free PMC article. Updated. Preprint.
-
Ribose-Map: a bioinformatics toolkit to map ribonucleotides embedded in genomic DNA.Nucleic Acids Res. 2019 Jan 10;47(1):e5. doi: 10.1093/nar/gky874. Nucleic Acids Res. 2019. PMID: 30272244 Free PMC article.
-
Two RNase H2 Mutants with Differential rNMP Processing Activity Reveal a Threshold of Ribonucleotide Tolerance for Embryonic Development.Cell Rep. 2018 Oct 30;25(5):1135-1145.e5. doi: 10.1016/j.celrep.2018.10.019. Cell Rep. 2018. PMID: 30380406 Free PMC article.
-
RNases H1 and H2: guardians of the stability of the nuclear genome when supply of dNTPs is limiting for DNA synthesis.Curr Genet. 2020 Dec;66(6):1073-1084. doi: 10.1007/s00294-020-01086-8. Epub 2020 Sep 4. Curr Genet. 2020. PMID: 32886170 Free PMC article. Review.
-
Aicardi-Goutières syndrome: clues from the RNase H2 knock-out mouse.J Mol Med (Berl). 2013 Nov;91(11):1235-40. doi: 10.1007/s00109-013-1061-x. Epub 2013 Jun 7. J Mol Med (Berl). 2013. PMID: 23744109 Review.
Cited by
-
Human ribomes reveal DNA-embedded ribonucleotides as a new type of epigenetic mark.bioRxiv [Preprint]. 2025 Jun 30:2025.06.27.661996. doi: 10.1101/2025.06.27.661996. bioRxiv. 2025. PMID: 40631190 Free PMC article. Preprint.
-
Uridine Embedded within DNA is Repaired by Uracil DNA Glycosylase via a Mechanism Distinct from That of Ribonuclease H2.J Am Chem Soc. 2025 Apr 2;147(13):11574-11583. doi: 10.1021/jacs.5c01436. Epub 2025 Mar 25. J Am Chem Soc. 2025. PMID: 40130361
References
-
- Fazzi E., Cattalini M., Orcesi S., Tincani A., Andreoli L., Balottin U., De Simone M., Fredi M., Facchetti F., Galli J., et al. Aicardi–Goutieres syndrome, a rare neurological disease in children: A new autoimmune disorder? Autoimmun. Rev. 2013;12:506–509. doi: 10.1016/J.AUTREV.2012.08.012. - DOI - PubMed
-
- Al Mutairi F., Alfadhel M., Nashabat M., El-Hattab A.W., Ben-Omran T., Hertecant J., Eyaid W., Ali R., Alasmari A., Kara M., et al. Phenotypic and Molecular Spectrum of Aicardi-Goutières Syndrome: A Study of 24 Patients. Pediatr. Neurol. 2018;78:35–40. doi: 10.1016/J.PEDIATRNEUROL.2017.09.002. - DOI - PubMed
-
- Crow Y.J., Massey R.F., Innes J.R., Pairaudeau P.W., Rowland Hill C.A., Woods C.G., Ali M., Livingston J.H., Lebon P., Nischall K., et al. Congenital glaucoma and brain stem atrophy as features of Aicardi–Goutières syndrome. Am. J. Med. Genet. 2004;129A:303–307. doi: 10.1002/AJMG.A.30250. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

