Microglial apolipoprotein E particles contribute to neuronal senescence and synaptotoxicity
- PMID: 38868202
- PMCID: PMC11167441
- DOI: 10.1016/j.isci.2024.110006
Microglial apolipoprotein E particles contribute to neuronal senescence and synaptotoxicity
Abstract
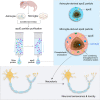
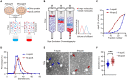
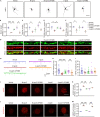
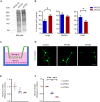
Apolipoprotein E (apoE) plays a crucial role in the pathogenesis of Alzheimer's disease (AD). Microglia exhibit a substantial upregulation of apoE in AD-associated circumstances, despite astrocytes being the primary source of apoE expression and secretion in the brain. Although the role of astrocytic apoE in the brain has been extensively investigated, it remains unclear that whether and how apoE particles generated from astrocytes and microglia differ in biological characteristic and function. Here, we demonstrate the differences in size between apoE particles generated from microglia and astrocytes. Microglial apoE particles impair neurite growth and synapses, and promote neuronal senescence, whereas depletion of GPNMB (glycoprotein non-metastatic melanoma protein B) in microglial apoE particles mitigated these deleterious effects. In addition, human APOE4-expressing microglia are more neurotoxic than APOE3-bearing microglia. For the first time, these results offer concrete evidence that apoE particles produced by microglia are involved in neuronal senescence and toxicity.
Keywords: Biological sciences; Cellular neuroscience; Neuroscience; Transcriptomics.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Differential Signaling Mediated by ApoE2, ApoE3, and ApoE4 in Human Neurons Parallels Alzheimer's Disease Risk.J Neurosci. 2019 Sep 11;39(37):7408-7427. doi: 10.1523/JNEUROSCI.2994-18.2019. Epub 2019 Jul 22. J Neurosci. 2019. PMID: 31331998 Free PMC article.
-
Expression and secretion of apoE isoforms in astrocytes and microglia during inflammation.Glia. 2021 Jun;69(6):1478-1493. doi: 10.1002/glia.23974. Epub 2021 Feb 8. Glia. 2021. PMID: 33556209 Free PMC article.
-
Isoform-Specific Effects of Apolipoprotein E on Markers of Inflammation and Toxicity in Brain Glia and Neuronal Cells In Vitro.Curr Issues Mol Biol. 2021 May 27;43(1):215-225. doi: 10.3390/cimb43010018. Curr Issues Mol Biol. 2021. PMID: 34071762 Free PMC article.
-
Understanding the Role of ApoE Fragments in Alzheimer's Disease.Neurochem Res. 2019 Jun;44(6):1297-1305. doi: 10.1007/s11064-018-2629-1. Epub 2018 Sep 17. Neurochem Res. 2019. PMID: 30225748 Review.
-
Astrocyte lipoproteins, effects of apoE on neuronal function, and role of apoE in amyloid-beta deposition in vivo.Microsc Res Tech. 2000 Aug 15;50(4):297-304. doi: 10.1002/1097-0029(20000815)50:4<297::AID-JEMT9>3.0.CO;2-C. Microsc Res Tech. 2000. PMID: 10936884 Review.
Cited by
-
The APOE-Microglia Axis in Alzheimer's Disease: Functional Divergence and Therapeutic Perspectives-A Narrative Review.Brain Sci. 2025 Jun 23;15(7):675. doi: 10.3390/brainsci15070675. Brain Sci. 2025. PMID: 40722268 Free PMC article. Review.
-
ApoE3 Christchurch and tau interaction as a protective mechanism against Alzheimer's disease.Alzheimers Dement. 2025 Jul;21(7):e70396. doi: 10.1002/alz.70396. Alzheimers Dement. 2025. PMID: 40637118 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

