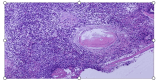
Beyond Midostaurin: Role of Avapritinib in Managing Systemic Mastocytosis
- PMID: 38868249
- PMCID: PMC11166541
- DOI: 10.7759/cureus.60161
Beyond Midostaurin: Role of Avapritinib in Managing Systemic Mastocytosis
Abstract
We present a case of an adult male who presented with pancytopenia accompanied by symptomatic anemia, necessitating chronic transfusions. He was diagnosed with systemic mastocytosis with an associated hematologic neoplasm. Following an inadequate response to midostaurin therapy, the patient was initiated on the newly approved avapritinib. The patient showed significant improvements in all three blood cell lines; however, he developed leg edema, blepharedema, and gum bleeding on this medication. This case underscores the intricacies of managing a patient with advanced systemic mastocytosis, the emerging role of highly selective KIT inhibition in its treatment, and the practical management of adverse medication effects.
Keywords: molecular target therapies; systemic mastocytosis; systemic mastocytosis with an associated clonal hematological non-mast cell lineage disease (sh-ahnmd); targeted therapeutics; tyrosine kinase receptor inhibitors.
Copyright © 2024, Pokima et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Novel approaches to treating advanced systemic mastocytosis.Clin Pharmacol. 2019 Jul 10;11:77-92. doi: 10.2147/CPAA.S206615. eCollection 2019. Clin Pharmacol. 2019. PMID: 31372066 Free PMC article. Review.
-
The Role of Avapritinib for the Treatment of Systemic Mastocytosis.Cureus. 2021 Sep 29;13(9):e18385. doi: 10.7759/cureus.18385. eCollection 2021 Sep. Cureus. 2021. PMID: 34729266 Free PMC article. Review.
-
Indirect treatment comparisons of avapritinib versus midostaurin for patients with advanced systemic mastocytosis.Future Oncol. 2022 Apr;18(13):1583-1594. doi: 10.2217/fon-2021-1509. Epub 2022 Feb 4. Future Oncol. 2022. PMID: 35114819
-
Response Criteria in Advanced Systemic Mastocytosis: Evolution in the Era of KIT Inhibitors.Int J Mol Sci. 2021 Mar 15;22(6):2983. doi: 10.3390/ijms22062983. Int J Mol Sci. 2021. PMID: 33804174 Free PMC article. Review.
-
Tyrosine Kinase Inhibitors in Non-advanced Systemic Mastocytosis.Immunol Allergy Clin North Am. 2023 Nov;43(4):743-750. doi: 10.1016/j.iac.2023.05.001. Epub 2023 Jun 20. Immunol Allergy Clin North Am. 2023. PMID: 37758410 Review.
Cited by
-
Breaking point: Systemic mastocytosis manifesting as severe osteoporosis.Oncoscience. 2025 Feb 4;12:13-20. doi: 10.18632/oncoscience.614. eCollection 2025. Oncoscience. 2025. PMID: 39911853 Free PMC article.
References
-
- Cutaneous manifestations in patients with mastocytosis: consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. Hartmann K, Escribano L, Grattan C, et al. J Allergy Clin Immunol. 2016;137:35–45. - PubMed
-
- Li W. Bisbane. Brisbane (AU): Exon Publications; 2022. The 5th Edition of the World Health Organization Classification of Hematolymphoid Tumors. Leukemia. - PubMed
-
- Epidemiology, prognosis, and risk factors in mastocytosis. Brockow K. Immunol Allergy Clin North Am. 2014;34:283–295. - PubMed
-
- Efficacy and safety of midostaurin in advanced systemic mastocytosis. Gotlib J, Kluin-Nelemans HC, George TI, et al. N Engl J Med. 2016;374:2530–2541. - PubMed
Publication types
LinkOut - more resources
Full Text Sources