Characterization in the rat of the individual tendency to rely on alcohol to cope with distress and the ensuing vulnerability to drink compulsively
- PMID: 38868300
- PMCID: PMC11168338
- DOI: 10.1093/braincomms/fcae169
Characterization in the rat of the individual tendency to rely on alcohol to cope with distress and the ensuing vulnerability to drink compulsively
Abstract
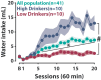
Only some vulnerable individuals who recreationally drink alcohol eventually develop the compulsive drinking pattern that characterizes alcohol use disorder. A new frontier in biomedical research lies in understanding the neurobehavioural mechanisms of this individual vulnerability, a necessary step towards developing novel effective therapeutic strategies. Translational research has been hindered by the lack of valid, reliable and robust approaches that enable the study of the influence of the reliance on alcohol to cope with stress or self-medicate negative emotional states on the subsequent transition to alcohol use disorder. We have therefore developed a behavioural task in the rat that enables the investigation of the neural and cellular basis of the exacerbation of the vulnerability to develop compulsive alcohol drinking by the use of alcohol to develop an adjunctive, anxiolytic, polydipsic drinking behaviour in a schedule-induced polydipsia procedure. Hence, in our task, alcohol is introduced in the schedule-induced polydipsia context after several weeks of training with water so that rats are exposed to alcohol for the first time in a distressing context and learn to drink alcohol as a coping strategy. Capitalizing on this protocol, we have consistently been able to identify a subpopulation of rats that were unable to learn to cope with negative states by drinking water and relied on alcohol to do so. This maladaptive reliance on alcohol drinking to cope with distress has been shown to be associated with an exacerbation of the subsequent transition to compulsive drinking. Furthermore, these vulnerable rats reached blood alcohol levels comparable to that of intoxication in humans, thereby developing two key features of alcohol use disorder, namely excessive alcohol intake and compulsive drinking. Altogether, this behavioural task provides a novel and unique tool for the investigation of the neurobehavioural mechanisms underlying the exacerbation of the individual vulnerability to developing compulsive alcohol drinking by the use of alcohol as a strategy to cope with distress, and for the evaluation of the efficacy of potential therapeutic strategies in a personalized medicine approach. This procedure, which focuses on an understudied but key factor of the development of alcohol use disorder, may become widely used as it benefits the fields of alcohol, emotion regulation and stress, the interest in which has substantially increased since the evidence of a profound exacerbation of alcohol use and alcohol-related negative consequences by the distress and social isolation engendered by the various measures implemented worldwide in response to the COVID-19 pandemic.
Keywords: alcohol; alcohol use disorder; individual vulnerability; schedule-induced polydipsia; self-medication.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures







Similar articles
-
Environment-dependent behavioral traits and experiential factors shape addiction vulnerability.Eur J Neurosci. 2021 Mar;53(6):1794-1808. doi: 10.1111/ejn.15087. Epub 2021 Jan 12. Eur J Neurosci. 2021. PMID: 33332672
-
The development of compulsive coping behavior depends on dorsolateral striatum dopamine-dependent mechanisms.Mol Psychiatry. 2023 Nov;28(11):4666-4678. doi: 10.1038/s41380-023-02256-z. Epub 2023 Sep 28. Mol Psychiatry. 2023. PMID: 37770577 Free PMC article.
-
The development of compulsive coping behaviour is associated with a downregulation of Arc in a Locus Coeruleus neuronal ensemble.Neuropsychopharmacology. 2023 Mar;48(4):653-663. doi: 10.1038/s41386-022-01522-y. Epub 2023 Jan 12. Neuropsychopharmacology. 2023. PMID: 36635597 Free PMC article.
-
Applications of schedule-induced polydipsia in rodents for the study of an excessive ethanol intake phenotype.Alcohol. 2014 May;48(3):265-76. doi: 10.1016/j.alcohol.2014.01.005. Epub 2014 Feb 28. Alcohol. 2014. PMID: 24680665 Free PMC article. Review.
-
Differential Neurobiological Markers in Phenotype-stratified Rats Modeling High or Low Vulnerability to Compulsive Behavior: A Narrative Review.Curr Neuropharmacol. 2023;21(9):1924-1933. doi: 10.2174/1570159X21666221121091454. Curr Neuropharmacol. 2023. PMID: 36411566 Free PMC article. Review.
References
-
- Gross JJ. Emotion regulation: Past, present, future. Cogn Emot. 1999;13(5):551–573.
-
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. - PubMed
-
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. - PubMed
-
- Gross JJ. Emotion regulation: Taking stock and moving forward. Emotion. 2013;13(3):359–365. - PubMed
-
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; 1984. xiii, 445 p.
LinkOut - more resources
Full Text Sources
