New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases
- PMID: 38868334
- PMCID: PMC10989969
- DOI: 10.1002/imt2.69
New insights into the mechanisms of high-fat diet mediated gut microbiota in chronic diseases
Abstract
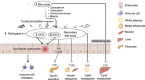
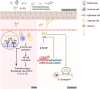
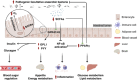
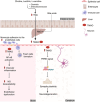
High-fat diet (HFD) has been recognized as a primary factor in the risk of chronic disease. Obesity, diabetes, gastrointestinal diseases, neurodegenerative diseases, and cardiovascular diseases have long been known as chronic diseases with high worldwide incidence. In this review, the influences of gut microbiota and their corresponding bacterial metabolites on the mechanisms of HFD-induced chronic diseases are systematically summarized. Gut microbiota imbalance is also known to increase susceptibility to diseases. Several studies have proven that HFD has a negative impact on gut microbiota, also exacerbating the course of many chronic diseases through increased populations of Erysipelotrichaceae, facultative anaerobic bacteria, and opportunistic pathogens. Since bile acids, lipopolysaccharide, short-chain fatty acids, and trimethylamine N-oxide have long been known as common features of bacterial metabolites, we will explore the possibility of synergistic mechanisms among those metabolites and gut microbiota in the context of HFD-induced chronic diseases. Recent literature concerning the mechanistic actions of HFD-mediated gut microbiota have been collected from PubMed, Google Scholar, and Scopus. The aim of this review is to provide new insights into those mechanisms and to point out the potential biomarkers of HFD-mediated gut microbiota.
Keywords: characteristic metabolites; chronic diseases; gut microbiota dysbiosis; high‐fat diet; targeted biomarkers.
© 2023 The Authors. iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Bacteroides fragilis aggravates high-fat diet-induced non-alcoholic fatty liver disease by regulating lipid metabolism and remodeling gut microbiota.Microbiol Spectr. 2024 Apr 2;12(4):e0339323. doi: 10.1128/spectrum.03393-23. Epub 2024 Feb 27. Microbiol Spectr. 2024. PMID: 38411057 Free PMC article.
-
Protective effect of agaro-oligosaccharides on gut dysbiosis and colon tumorigenesis in high-fat diet-fed mice.Am J Physiol Gastrointest Liver Physiol. 2016 Mar 15;310(6):G367-75. doi: 10.1152/ajpgi.00324.2015. Epub 2016 Jan 14. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 26767984
-
Ileal Bile Acid Transporter Inhibitor Improves Hepatic Steatosis by Ameliorating Gut Microbiota Dysbiosis in NAFLD Model Mice.mBio. 2021 Aug 31;12(4):e0115521. doi: 10.1128/mBio.01155-21. Epub 2021 Jul 6. mBio. 2021. PMID: 34225483 Free PMC article.
-
Dietary glycation compounds - implications for human health.Crit Rev Toxicol. 2024 Sep;54(8):485-617. doi: 10.1080/10408444.2024.2362985. Epub 2024 Aug 16. Crit Rev Toxicol. 2024. PMID: 39150724
-
Diet-mediated gut microbial community modulation and signature metabolites as potential biomarkers for early diagnosis, prognosis, prevention and stage-specific treatment of colorectal cancer.J Adv Res. 2023 Oct;52:45-57. doi: 10.1016/j.jare.2022.12.015. Epub 2022 Dec 31. J Adv Res. 2023. PMID: 36596411 Free PMC article. Review.
Cited by
-
Gut microbiota-mediated gut-liver axis: a breakthrough point for understanding and treating liver cancer.Clin Mol Hepatol. 2025 Apr;31(2):350-381. doi: 10.3350/cmh.2024.0857. Epub 2024 Dec 11. Clin Mol Hepatol. 2025. PMID: 39659059 Free PMC article. Review.
-
Tryptophan Attenuates Chronic Restraint Stress-Induced Intestinal Injury Through Modulation of Intestinal Barrier Integrity and Gut Microbiota Homeostasis.Nutrients. 2025 Mar 11;17(6):975. doi: 10.3390/nu17060975. Nutrients. 2025. PMID: 40290020 Free PMC article.
-
Dietary therapies interlinking with gut microbes toward human health: Past, present, and future.Imeta. 2024 Aug 10;3(5):e230. doi: 10.1002/imt2.230. eCollection 2024 Oct. Imeta. 2024. PMID: 39429878 Free PMC article.
-
Multi-View Integrative Approach For Imputing Short-Chain Fatty Acids and Identifying Key factors predicting Blood SCFA.bioRxiv [Preprint]. 2024 Sep 27:2024.09.25.614767. doi: 10.1101/2024.09.25.614767. bioRxiv. 2024. PMID: 39386638 Free PMC article. Preprint.
-
High-fat diet may increase the risk of insulin resistance by inducing dysbiosis.Metabol Open. 2025 Jul 22;27:100381. doi: 10.1016/j.metop.2025.100381. eCollection 2025 Sep. Metabol Open. 2025. PMID: 40741424 Free PMC article. Review.
References
-
- Imamura, Fumiaki , Micha Renata, Khatibzadeh Shahab, Fahimi Saman, Shi Peilin, Powles John, and Mozaffarian Dariush. 2015. “Dietary Quality Among Men and Women In 187 Countries In 1990 and 2010: A Systematic Assessment.” The Lancet Global Health 3: e132–42. 10.1016/S2214-109X(14)70381-X - DOI - PMC - PubMed
-
- Więckowska‐Gacek, Angelika , Mietelska‐Porowska Anna, Wydrych Małgorzata, and Wojda Urszula. 2021. “Western Diet as a Trigger of Alzheimer's Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration.” Ageing Research Reviews 70: 101397. 10.1016/j.arr.2021.101397 - DOI - PubMed
-
- Myette‐Côté, Étienne , Durrer Cody, Neudorf Helena, Bammert Tyler D., Botezelli José Diego, Johnson James D., DeSouza Christopher A., and Little Jonathan P.. 2018. “The Effect of a Short‐Term Low‐Carbohydrate, High‐Fat Diet With or Without Postmeal Walks on Glycemic Control and Inflammation in Type 2 Diabetes: A Randomized Trial.” American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology 315, R1210–19. 10.1152/ajpregu.00240.2018 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
