MBPD: A multiple bacterial pathogen detection pipeline for One Health practices
- PMID: 38868336
- PMCID: PMC10989770
- DOI: 10.1002/imt2.82
MBPD: A multiple bacterial pathogen detection pipeline for One Health practices
Abstract
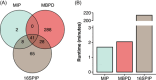
Bacterial pathogens are one of the major threats to biosafety and environmental health, and advanced assessment is a prerequisite to combating bacterial pathogens. Currently, 16S rRNA gene sequencing is efficient in the open-view detection of bacterial pathogens. However, the taxonomic resolution and applicability of this method are limited by the domain-specific pathogen database, taxonomic profiling method, and sequencing target of 16S variable regions. Here, we present a pipeline of multiple bacterial pathogen detection (MBPD) to identify the animal, plant, and zoonotic pathogens. MBPD is based on a large, curated database of the full-length 16S genes of 1986 reported bacterial pathogen species covering 72,685 sequences. In silico comparison allowed MBPD to provide the appropriate similarity threshold for both full-length and variable-region sequencing platforms, while the subregion of V3-V4 (mean: 88.37%, accuracy rate compared to V1-V9) outperformed other variable regions in pathogen identification compared to full-length sequencing. Benchmarking on real data sets suggested the superiority of MBPD in a broader range of pathogen detections compared with other methods, including 16SPIP and MIP. Beyond detecting the known causal agent of animal, human, and plant diseases, MBPD is capable of identifying cocontaminating pathogens from biological and environmental samples. Overall, we provide a MBPD pipeline for agricultural, veterinary, medical, and environmental monitoring to achieve One Health.
Keywords: 16S rRNA gene sequencing; One Health; bacterial pathogen detection.
© 2023 The Authors. iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Comparison of the full-length sequence and sub-regions of 16S rRNA gene for skin microbiome profiling.mSystems. 2024 Jul 23;9(7):e0039924. doi: 10.1128/msystems.00399-24. Epub 2024 Jun 27. mSystems. 2024. PMID: 38934545 Free PMC article.
-
Determining the most accurate 16S rRNA hypervariable region for taxonomic identification from respiratory samples.Sci Rep. 2023 Mar 9;13(1):3974. doi: 10.1038/s41598-023-30764-z. Sci Rep. 2023. PMID: 36894603 Free PMC article.
-
Primer, Pipelines, Parameters: Issues in 16S rRNA Gene Sequencing.mSphere. 2021 Feb 24;6(1):e01202-20. doi: 10.1128/mSphere.01202-20. mSphere. 2021. PMID: 33627512 Free PMC article.
-
Species-level resolution for the vaginal microbiota with short amplicons.mSystems. 2024 Feb 20;9(2):e0103923. doi: 10.1128/msystems.01039-23. Epub 2024 Jan 26. mSystems. 2024. PMID: 38275296 Free PMC article.
-
GSR-DB: a manually curated and optimized taxonomical database for 16S rRNA amplicon analysis.mSystems. 2024 Feb 20;9(2):e0095023. doi: 10.1128/msystems.00950-23. Epub 2024 Jan 8. mSystems. 2024. PMID: 38189256 Free PMC article.
Cited by
-
Technologies for detecting biological risk factors in agricultural products and their applications.Curr Res Food Sci. 2025 May 6;10:101068. doi: 10.1016/j.crfs.2025.101068. eCollection 2025. Curr Res Food Sci. 2025. PMID: 40485901 Free PMC article. Review.
-
Bacterial population dynamics and biosafety assessment in a closed Bioregenerative Life Support System during the "Lunar Palace 365" experiment.Sci China Life Sci. 2025 Jul;68(7):2137-2149. doi: 10.1007/s11427-024-2857-1. Epub 2025 May 13. Sci China Life Sci. 2025. PMID: 40555951
-
Molecular assessment of oyster microbiomes and viromes reveals their potential as pathogen and ecological sentinels.One Health. 2025 Jan 13;20:100973. doi: 10.1016/j.onehlt.2025.100973. eCollection 2025 Jun. One Health. 2025. PMID: 39898315 Free PMC article.
-
A Metagenomic Investigation of Potential Health Risks and Element Cycling Functions of Bacteria and Viruses in Wastewater Treatment Plants.Viruses. 2024 Mar 29;16(4):535. doi: 10.3390/v16040535. Viruses. 2024. PMID: 38675877 Free PMC article.
-
Plant Diversity Reduces the Risk of Antibiotic Resistance Genes in Agroecosystems.Adv Sci (Weinh). 2025 Mar;12(11):e2410990. doi: 10.1002/advs.202410990. Epub 2025 Jan 28. Adv Sci (Weinh). 2025. PMID: 39874208 Free PMC article.
References
-
- Gruetzmacher, Kim , Karesh William B., Amuasi John H., Arshad Adnan, Farlow Andrew, Gabrysch Sabine, Jetzkowitz Jens, et al. 2021. “The Berlin Principles on One Health—Bridging Global Health and Conservation.” Science of The Total Environment 764: 142919. 10.1016/j.scitotenv.2020.142919 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
