Adeno-associated virus perfusion enhanced expression: A commercially scalable, high titer, high quality producer cell line process
- PMID: 38868441
- PMCID: PMC11166877
- DOI: 10.1016/j.omtm.2024.101266
Adeno-associated virus perfusion enhanced expression: A commercially scalable, high titer, high quality producer cell line process
Abstract
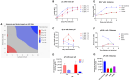
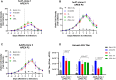
With safety and efficacy demonstrated over hundreds of clinical trials in the last 30 years, along with at least six recent global marketing authorizations achieved since 2017, recombinant adeno-associated viruses (rAAVs) have been established as the leading therapeutic gene transfer vector for rare, monogenic diseases. Significant advances in manufacturing technology have been made in the last few decades to address challenges with GMP production of rAAV products, although yield, cost, scalability, and quality remain a challenge. With transient transfection processes established as a manufacturing platform for multiple commercial AAV products, there remains significant yield, cost, robustness, and scalability constraints that need to be resolved to enable a reliable supply of rAAV products for global patient access. The development of stable producer cell lines for rAAV products has enabled scalability and, in some cases, improvements in productivity. Herein we describe a novel AAV perfusion-enhanced expression (APEX) process, resulting in higher maximum cell densities in the production bioreactor with a 3- to 6-fold increase in volumetric productivity. This process has been successfully demonstrated across multiple serotypes in large scale cell culture with titers approaching 1 × 1012 GC/mL. The APEX production platform marks a significant leap forward in the efficient and effective manufacturing of rAAV vector products.
Keywords: AAV; cell density effect; cost of production; high-yield; perfusion; process development; producer cell line; scalable.
© 2024 Ultragenyx Pharmaceutical Inc.
Conflict of interest statement
This work was funded by Ultragenyx Pharmaceuticals. All authors were employed by Ultragenyx Pharmaceuticals when the experiments were executed. Several authors are shareholders of Ultragenyx Pharmaceuticals. The work is related to patent WO2023172491A1 - Modified batch aav production systems and methods.
Figures






References
-
- Sha S., Maloney A.J., Katsikis G., Nguyen T.N.T., Neufeld C., Wolfrum J., Barone P.W., Springs S.L., Manalis S.R., Sinskey A.J., Braatz R.D. Cellular pathways of recombinant adeno-associated virus production for gene therapy. Biotechnol. Adv. 2021;49 doi: 10.1016/j.biotechadv.2021.107764. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

