Black rice diet alleviates colorectal cancer development through modulating tryptophan metabolism and activating AHR pathway
- PMID: 38868519
- PMCID: PMC10989083
- DOI: 10.1002/imt2.165
Black rice diet alleviates colorectal cancer development through modulating tryptophan metabolism and activating AHR pathway
Erratum in
-
Correction to "Black rice diet alleviates colorectal cancer development through modulating tryptophan metabolism and activating AHR pathway".Imeta. 2025 Apr 29;4(3):e70039. doi: 10.1002/imt2.70039. eCollection 2025 Jun. Imeta. 2025. PMID: 40469519 Free PMC article.
Abstract
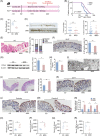
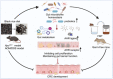
Consumption of dietary fiber and anthocyanin has been linked to a lower incidence of colorectal cancer (CRC). This study scrutinizes the potential antitumorigenic attributes of a black rice diet (BRD), abundantly rich in dietary fiber and anthocyanin. Our results demonstrate notable antitumorigenic effects in mice on BRD, indicated by a reduction in both the size and number of intestinal tumors and a consequent extension in life span, compared to control diet-fed counterparts. Furthermore, fecal transplants from BRD-fed mice to germ-free mice led to a decrease in colonic cell proliferation, coupled with maintained integrity of the intestinal barrier. The BRD was associated with significant shifts in gut microbiota composition, specifically an augmentation in probiotic strains Bacteroides uniformis and Lactobacillus. Noteworthy changes in gut metabolites were also documented, including the upregulation of indole-3-lactic acid and indole. These metabolites have been identified to stimulate the intestinal aryl hydrocarbon receptor pathway, inhibiting CRC cell proliferation and colorectal tumorigenesis. In summary, these findings propose that a BRD may modulate the progression of intestinal tumors by fostering protective gut microbiota and metabolite profiles. The study accentuates the potential health advantages of whole-grain foods, emphasizing the potential utility of black rice in promoting health.
Keywords: black rice diet; colorectal cancer; gut metabolites; gut microbiome.
© 2024 The Authors. iMeta published by John Wiley & Sons Australia, Ltd on behalf of iMeta Science.
Conflict of interest statement
The authors have declared no competing interests.
Figures




References
-
- Arima, Kota , Zhong Rong, Ugai Tomotaka, Zhao Melissa, Haruki Koichiro, Akimoto Naohiko, Lau Mai Chan, et al. 2022. “Western‐Style Diet, Pks Island‐Carrying Escherichia coli, and Colorectal Cancer: Analyses From Two Large Prospective Cohort Studies.” Gastroenterology 163: 862–874. 10.1053/j.gastro.2022.06.054 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
