Pulmonary hypertension in the intensive care unit after pediatric allogeneic hematopoietic stem cell transplant: incidence, risk factors, and outcomes
- PMID: 38868534
- PMCID: PMC11167102
- DOI: 10.3389/fonc.2024.1415984
Pulmonary hypertension in the intensive care unit after pediatric allogeneic hematopoietic stem cell transplant: incidence, risk factors, and outcomes
Abstract
Objective: To determine the incidence, risk factors, and outcomes of pulmonary hypertension (PH) in the pediatric intensive care unit (PICU) after pediatric hematopoietic stem cell transplant (HCT).
Methods: This was a retrospective study of pediatric patients who underwent allogeneic HCT between January 2008-December 2014 at a center contributing to the Center for International Blood and Marrow Transplant Research data registry. Incidence of PH was assessed from PICU diagnostic codes from records merged from the Virtual Pediatric Systems database. Regression and survival analyses identified factors associated with post-HCT PH. Additional post-HCT morbidities and survival after PH were also assessed.
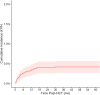
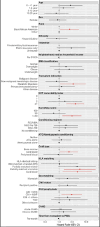
Results: Among 6,995 HCT recipients, there were 29 cases of PH, a cumulative incidence of 0.42% (95% CI 0.27%-0.57%) at 60 months post-HCT. In the sub-cohort of 1,067 patients requiring intensive care after HCT, this accounted for a PH prevalence of 2.72% (95% CI 1.74-3.69%). There was an increased risk of developing PH associated with Black/African American race, metabolic disorders, partially HLA-matched or cord blood allografts, graft-versus-host prophylaxis regimen, and lower pre-HCT functional status. Patients who developed PH had significant PICU comorbidities including heart failure, pulmonary hemorrhage, respiratory failure, renal failure, and infections. Survival at 6 months after diagnosis of post-HCT PH was 51.7% (95% CI 32.5%-67.9%).
Conclusions: PH is a rare but serious complication in the pediatric post-HCT population. A significant burden of additional comorbidities, procedural interventions, and risk of mortality is associated with its development. Close monitoring and prompt intervention for this severe complication are necessary in this vulnerable population.
Keywords: critical care; pediatrics; pulmonary hypertension; pulmonary vascular disease; stem cell transplant.
Copyright © 2024 Smith, Cheng, Phelan, Brazauskas, Strom, Ahn, Hamilton, Peterson, Savani, Schoemans, Schoettler, Sorror, Keller, Higham, Dvorak, Fineman and Zinter.
Conflict of interest statement
RP reports bluebird bio: advisory board and Amgen: research funding. BH reports ad hoc advisory boards for Nkarta, Sanofi, Incyte, Rigel, Maat; consultancy with ACI Group, Therakos/Mallinkrodt speaker fees; data safety monitoring committee for Angiocrine; adjudication committee with CSL Behring. HS reports having received personal fees from Incyte, Janssen, Novartis, Sanofi and from the Belgian Hematological Society BHS paid to her institution; and serves as a volunteer for the EBMT. MSc reports consulting and honorarium from Omeros and Alexion. MSo reports receiving honoraria from JAZZ Pharmaceuticals Canada and research funding per a contract with Massachusetts General Hospital. CD reports consulting for Alexion and Jazz Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Critical Illness Risk and Long-Term Outcomes Following Intensive Care in Pediatric Hematopoietic Cell Transplant Recipients.medRxiv [Preprint]. 2023 Aug 5:2023.07.31.23293444. doi: 10.1101/2023.07.31.23293444. medRxiv. 2023. Update in: Blood Adv. 2024 Feb 27;8(4):1002-1017. doi: 10.1182/bloodadvances.2023011002. PMID: 37577706 Free PMC article. Updated. Preprint.
-
Comprehensive Prognostication in Critically Ill Pediatric Hematopoietic Cell Transplant Patients: Results from Merging the Center for International Blood and Marrow Transplant Research (CIBMTR) and Virtual Pediatric Systems (VPS) Registries.Biol Blood Marrow Transplant. 2020 Feb;26(2):333-342. doi: 10.1016/j.bbmt.2019.09.027. Epub 2019 Sep 26. Biol Blood Marrow Transplant. 2020. PMID: 31563573 Free PMC article.
-
Risk factors predicting need for the pediatric intensive care unit (PICU) post-hematopoietic cell transplant, PICU utilization, and outcomes following HCT: a single center retrospective analysis.Front Pediatr. 2024 Apr 16;12:1385153. doi: 10.3389/fped.2024.1385153. eCollection 2024. Front Pediatr. 2024. PMID: 38690520 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Medical emergencies in pediatric blood & marrow transplant and cellular therapies.Front Pediatr. 2023 Feb 7;11:1075644. doi: 10.3389/fped.2023.1075644. eCollection 2023. Front Pediatr. 2023. PMID: 36824648 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

