Digitoxose as powerful glycosyls for building multifarious glycoconjugates of natural products and un-natural products
- PMID: 38868608
- PMCID: PMC11167396
- DOI: 10.1016/j.synbio.2024.05.012
Digitoxose as powerful glycosyls for building multifarious glycoconjugates of natural products and un-natural products
Abstract
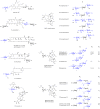
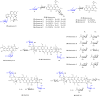
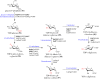
Digitoxose, a significant 2,6-dideoxyhexose found in nature, exists in many small-molecule natural products. These digitoxose-containing natural products can be divided into steroids, macrolides, macrolactams, anthracyclines, quinones, enediynes, acyclic polyene, indoles and oligosaccharides, that exhibit antibacterial, anti-viral, antiarrhythmic, and antitumor activities respectively. As most of digitoxose-containing natural products for clinical application or preclinical tests, this review also summarizes the biosynthesis of digitoxose, and application of compound diversification by introducing sugar plasmids. It may provide a practical approach to expanding the diversity of digitoxose-containing products.
Keywords: Biosynthesis; Digitoxose; Digitoxose glycosylation; Small-molecule natural products.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Characterization of the sugar-O-methyltransferase LobS1 in lobophorin biosynthesis.Appl Microbiol Biotechnol. 2013 Oct;97(20):9043-53. doi: 10.1007/s00253-013-5083-7. Epub 2013 Jul 20. Appl Microbiol Biotechnol. 2013. PMID: 23868295
-
Efficient synthesis of the deoxysugar part of versipelostatin by direct and stereoselective glycosylation and revision of the structure of the trisaccharide unit.Chem Asian J. 2009 Jul 6;4(7):1114-25. doi: 10.1002/asia.200800448. Chem Asian J. 2009. PMID: 19347890
-
Biosynthesis and pathway engineering of antifungal polyene macrolides in actinomycetes.J Ind Microbiol Biotechnol. 2013 Jun;40(6):529-43. doi: 10.1007/s10295-013-1258-6. Epub 2013 Mar 21. J Ind Microbiol Biotechnol. 2013. PMID: 23515854 Review.
-
Biosynthesis of Lincosamide Antibiotics: Reactions Associated with Degradation and Detoxification Pathways Play a Constructive Role.Acc Chem Res. 2018 Jun 19;51(6):1496-1506. doi: 10.1021/acs.accounts.8b00135. Epub 2018 May 24. Acc Chem Res. 2018. PMID: 29792672 Review.
-
Synthesis of the α-Linked Digitoxose Trisaccharide Fragment of Kijanimicin: An Unexpected Application of Glycosyl Sulfonates.Org Lett. 2022 Jan 21;24(2):731-735. doi: 10.1021/acs.orglett.1c04190. Epub 2022 Jan 10. Org Lett. 2022. PMID: 35005969
Cited by
-
Saarvienin A-A Novel Glycopeptide with Potent Activity against Drug-Resistant Bacteria.Angew Chem Int Ed Engl. 2025 Jun 17;64(25):e202425588. doi: 10.1002/anie.202425588. Epub 2025 Apr 25. Angew Chem Int Ed Engl. 2025. PMID: 40249031 Free PMC article.
References
-
- Al-Fadhli A.A., Threadgill M.D., Mohammed F., Sibley P., Al-Ariqi W., Parveen I. Macrolides from rare actinomycetes: structures and bioactivities. Int J Antimicrob Agents. 2022;59(2) - PubMed
-
- Furneri P.M., Nicoletti G. Macrolides: present and future. An appraisal of in-vitro activity and pharmacokinetic behavior. J Chemother. 1991;3(Suppl 1):24–27. - PubMed
-
- Kiyoshima K., Takada K., Yamamoto M., Kubo K., Okamoto R., Fukagawa Y., et al. New tylosin analogs produced by mutants of Streptomyces fradiae. J Antibiot (Tokyo) 1987;40(8):1123–1130. - PubMed
-
- Murakami R., Tomikawa T., Shin-ya K., Shinozaki J., Kajiura T., Seto H., et al. Ammocidin, a new apoptosis inducer in ras-dependent cells from Saccharothrix sp. II. Physico-chemical properties and structure elucidation. J Antibiot (Tokyo) 2001;54(9):714–717. - PubMed
-
- Murakami R., Shinozaki J., Kajiura T., Kozone I., Takagi M., Shin-Ya K., et al. Ammocidins B, C and D, new cytotoxic 20-membered macrolides from Saccharothrix sp. AJ9571. J Antibiot (Tokyo) 2009;62(3):123–127. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

