18‑α‑glycyrrhetinic acid induces apoptosis in gingival fibroblasts exposed to phenytoin
- PMID: 38868612
- PMCID: PMC11168035
- DOI: 10.3892/etm.2024.12586
18‑α‑glycyrrhetinic acid induces apoptosis in gingival fibroblasts exposed to phenytoin
Abstract
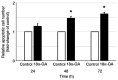
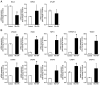
Phenytoin (PHT)-induced gingival overgrowth is caused by the increased proliferation and reduced apoptosis of gingival fibroblasts in inflammatory gingiva. Licorice has long been used as a component of therapeutic preparations. It inhibits cell proliferation, induces cell apoptosis and has anti-inflammatory effects. 18-α-glycyrrhetinic acid (18α-GA), the active compound in licorice, promotes apoptosis in various types of cells. The present study determined whether 18α-GA affects apoptosis in gingival fibroblasts exposed to PHT. The present study aimed to establish a basis for the therapeutic application of 18α-GA to treat the gingival overgrowth induced by PHT. Human gingival fibroblasts from healthy donors were cultured to semi-confluence and then stimulated in serum-free DMEM containing PHT with or without 18α-GA for subsequent experiments. Apoptotic cells were detected by ELISA. Analysis of the distribution of cell cycle phases and the apoptotic cell population was performed by flow cytometry. The expression levels of mRNAs and proteins of apoptotic regulators were measured using reverse transcription-quantitative PCR and western blotting, respectively. Caspase (CASP) activities were assessed by an ELISA. Treatment with 18α-GA markedly increased the number of apoptotic cells, reduced BCL2 mRNA expression, increased CASP2 and receptor (TNFRSF)-interacting serine-threonine kinase 1 (RIPK1) domain containing adaptor with death domain, Fas (TNFRSF6)-associated via death domain, RIPK1, tumor necrosis factor receptor superfamily; member 1A, TNF receptor-associated factor 2, CASP2, CASP3 and CASP9 mRNA expression, and also upregulated the protein expression levels and activities of caspase-2, caspase-3 and caspase-9. These results demonstrated that 18α-GA induced apoptosis through the activation of the Fas and TNF pathways in the death receptor signaling pathway in gingival fibroblasts treated with PHT. 18α-GA exhibited therapeutic potential for the treatment of PHT-induced gingival overgrowth.
Keywords: 18α-GA; PHT; apoptosis; death receptor pathway; gingival fibroblast; gingival overgrowth.
Copyright: © 2024 Takeuchi et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
Phenytoin-induced gingival overgrowth caused by death receptor pathway malfunction.Oral Dis. 2017 Jul;23(5):653-659. doi: 10.1111/odi.12651. Epub 2017 Mar 24. Oral Dis. 2017. PMID: 28160766
-
Possible pharmacotherapy for nifedipine-induced gingival overgrowth: 18α-glycyrrhetinic acid inhibits human gingival fibroblast growth.Br J Pharmacol. 2016 Mar;173(5):913-24. doi: 10.1111/bph.13410. Epub 2016 Feb 3. Br J Pharmacol. 2016. PMID: 26676684 Free PMC article.
-
Inhibition of G₁ cell cycle arrest in human gingival fibroblasts exposed to phenytoin.Fundam Clin Pharmacol. 2014 Feb;28(1):114-9. doi: 10.1111/j.1472-8206.2012.01065.x. Epub 2012 Aug 14. Fundam Clin Pharmacol. 2014. PMID: 22888954
-
18α-Glycyrrhetinic Acid Induces Apoptosis of HL-60 Human Leukemia Cells through Caspases- and Mitochondria-Dependent Signaling Pathways.Molecules. 2016 Jul 1;21(7):872. doi: 10.3390/molecules21070872. Molecules. 2016. PMID: 27376261 Free PMC article.
-
18α-glycyrrhetinic acid extracted from Glycyrrhiza radix inhibits proliferation and promotes apoptosis of the hepatic stellate cell line.J Dig Dis. 2013 Jun;14(6):328-36. doi: 10.1111/1751-2980.12041. J Dig Dis. 2013. PMID: 23362936
Cited by
-
Cyclosporine A Causes Gingival Overgrowth by Promoting Entry into the S Phase at the G1/S Cell Cycle Checkpoint in Gingival Fibroblasts Exposed to Lipopolysaccharide.Diseases. 2024 Dec 10;12(12):322. doi: 10.3390/diseases12120322. Diseases. 2024. PMID: 39727652 Free PMC article.
-
Cyclosporine A causes gingival overgrowth via reduced G1 cell cycle arrest in gingival fibroblasts.PLoS One. 2024 Dec 20;19(12):e0309189. doi: 10.1371/journal.pone.0309189. eCollection 2024. PLoS One. 2024. PMID: 39705288 Free PMC article.
References
-
- Takeuchi R, Matsumoto H, Okada H, Hori M, Gunji A, Hakozaki K, Akimoto Y, Fujii A. Differences of cell growth and cell cycle regulators induced by basic fibroblast growth factor between nifedipine responders and non-responders. J Pharmacol Sci. 2007;103:168–174. doi: 10.1254/jphs.fp0060928. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
