Anti-Leishmanial Effects of a Novel Biocompatible Non-Invasive Nanofibers Containing Royal Jelly and Propolis against Iranian Strain of Leishmania major (MRHO/IR/75/ER): an In-Vitro Study
- PMID: 38868671
- PMCID: PMC11164616
- DOI: 10.18502/jad.v17i4.15294
Anti-Leishmanial Effects of a Novel Biocompatible Non-Invasive Nanofibers Containing Royal Jelly and Propolis against Iranian Strain of Leishmania major (MRHO/IR/75/ER): an In-Vitro Study
Abstract
Background: Current medications especially the pentavalent antimonial compounds have been used as the first line treatment of cutaneous leishmaniasis (CL), but they have limitations due to serious side effects such as drug resistance, cardio and nephrotoxicity, and high costs. Hence, the demand to find more usable drugs is evident. Synthesis and development of natural, effective, biocompatible, and harmless compounds against Leishmania major is the principal priority of this study.
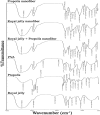
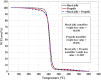
Methods: By electrospinning method, a new type of nanofiber were synthesized from royal jelly and propolis with different ratios. Nanofibers were characterized by Scanning Electron Microscope (SEM), Transmission Electron Microscopy (TEM), Thermogravimetric Analysis (TGA), Contact angle, and Fourier-transform infrared spectroscopy (FTIR). The Half-maximal inhibitory concentration (IC50), Half-maximal effective concentration (EC50) and the 50% cytotoxic concentration (CC50) for different concentrations of nanofibers were determined using quantitative calorimetric methods. Inductively coupled plasma-optical emission spectrometry (ICP-OES) and flow cytometry were performed as complementary tests.
Results: The results showed that the proposed formulas provide a new achievement that, despite the significant killing activity on L. major, has negligible cytotoxicity on the host cells. Royal jelly nanofibers have significantly shown the best 72 hours results (IC50= 35 μg/ml and EC50=16.4 μg/ml) and the least cytotoxicity.
Conclusion: This study presents a great challenge to introduce a new low-cost treatment method for CL, accelerate wound healing, and reduce scarring with minimal side effects and biocompatible materials. Royal jelly and propolis nanofibers significantly inhibit the growth of L. major in-vitro.
Keywords: In-vitro; Leishmania major; Nanofiber; Propolis; Royal jelly.
Copyright © 2023 The Authors. Published by Tehran University of Medical Sciences.
Figures










References
-
- Nabas Z, Haddadin MS, Haddadin J, Nazer IK. (2014) Chemical composition of royal jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Pol J Food Nutr Sci. 64(3): 171–180.
LinkOut - more resources
Full Text Sources
