CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis
- PMID: 38869035
- PMCID: PMC11569450
- DOI: 10.1177/23969873241260538
CSF and plasma biomarkers in cerebral amyloid angiopathy: A single-center study and a systematic review/meta-analysis
Abstract
Introduction: There are limited data regarding cerebrospinal fluid (CSF) and plasma biomarkers among patients with Cerebral Amyloid Angiopathy (CAA). We sought to investigate the levels of four biomarkers [β-amyloids (Aβ42 and Aβ40), total tau (tau) and phosphorylated tau (p-tau)] in CAA patients compared to healthy controls (HC) and patients with Alzheimer Disease (AD).
Patients and methods: A systematic review and meta-analysis of published studies, including also a 5 year single-center cohort study, with available data on CSF and plasma biomarkers in symptomatic sporadic CAA versus HC and AD was conducted. Biomarkers' comparisons were investigated using random-effects models based on the ratio of mean (RoM) biomarker concentrations. RoM < 1 and RoM > 1 indicate lower and higher biomarker concentration in CAA compared to another population, respectively.
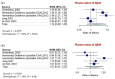
Results: We identified nine cohorts, comprising 327 CAA patients (mean age: 71 ± 5 years; women: 45%) versus 336 HC (mean age: 65 ± 5 years; women: 45%) and 384 AD patients (mean age: 68 ± 3 years; women: 53%) with available data on CSF biomarkers. CSF Aβ42 levels [RoM: 0.47; 95% CI: 0.36-0.62; p < 0.0001], Aβ40 levels [RoM: 0.70; 95% CI: 0.63-0.79; p < 0.0001] and the ratio Aβ42/Aβ40 [RoM: 0.62; 95% CI: 0.39-0.98; p = 0.0438] differentiated CAA from HC. CSF Aβ40 levels [RoM: 0.73; 95% CI: 0.64-0.83; p = 0.0003] differentiated CAA from AD. CSF tau and p-tau levels differentiated CAA from HC [RoM: 1.71; 95% CI: 1.41-2.09; p = 0.0002 and RoM: 1.44; 95% CI: 1.20-1.73; p = 0.0014, respectively] and from AD [RoM: 0.65; 95% CI: 0.58-0.72; p < 0.0001 and RoM: 0.64; 95% CI: 0.57-0.71; p < 0.0001, respectively]. Plasma Aβ42 [RoM: 1.14; 95% CI: 0.89-1.45; p = 0.2079] and Aβ40 [RoM: 1.07; 95% CI: 0.91-1.25; p = 0.3306] levels were comparable between CAA and HC.
Conclusions: CAA is characterized by a distinct CSF biomarker pattern compared to HC and AD. CSF Aβ40 levels are lower in CAA compared to HC and AD, while tau and p-tau levels are higher in CAA compared to HC, but lower in comparison to AD patients.
Keywords: Cerebral amyloid angiopathy; amyloid-β; biomarkers; cerebrospinal fluid; plasma; tau-protein.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001; 56: 537–539. - PubMed
-
- Malhotra K, Theodorou A, Katsanos AH, et al. Prevalence of clinical and neuroimaging markers in cerebral amyloid angiopathy: a systematic review and meta-analysis. Stroke 2022; 53: 1944–1953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

