The tryptophan metabolic pathway of the microbiome and host cells in health and disease
- PMID: 38869080
- PMCID: PMC11562643
- DOI: 10.1093/intimm/dxae035
The tryptophan metabolic pathway of the microbiome and host cells in health and disease
Abstract
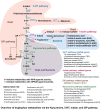
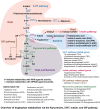
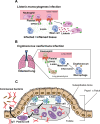
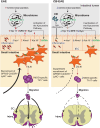
The intricate and dynamic tryptophan (Trp) metabolic pathway in both the microbiome and host cells highlights its profound implications for health and disease. This pathway involves complex interactions between host cellular and bacteria processes, producing bioactive compounds such as 5-hydroxytryptamine (5-HT) and kynurenine derivatives. Immune responses to Trp metabolites through specific receptors have been explored, highlighting the role of the aryl hydrocarbon receptor in inflammation modulation. Dysregulation of this pathway is implicated in various diseases, such as Alzheimer's and Parkinson's diseases, mood disorders, neuronal diseases, autoimmune diseases such as multiple sclerosis (MS), and cancer. In this article, we describe the impact of the 5-HT, Trp, indole, and Trp metabolites on health and disease. Furthermore, we review the impact of microbiome-derived Trp metabolites that affect immune responses and contribute to maintaining homeostasis, especially in an experimental autoimmune encephalitis model of MS.
Keywords: GPR35; brain disease; immune cell; kynurenic acid.
© The Author(s) 2024. Published by Oxford University Press on behalf of The Japanese Society for Immunology.
Figures




References
-
- Agus A, Planchais J, Sokol H.. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23:716–24. https://doi.org/10.1016/j.chom.2018.05.003 - DOI - PubMed
-
- Liu M, Nieuwdorp M, de Vos WM, et al.Microbial tryptophan metabolism tunes host immunity, metabolism, and extraintestinal disorders. Metabolites 2022;12:834. https://doi.org/10.3390/metabo12090834 - DOI - PMC - PubMed
-
- Hou Y, Li J, Ying S.. Tryptophan metabolism and gut microbiota: a novel regulatory axis integrating the microbiome, immunity, and cancer. Metabolites 2023;13:1166. https://doi.org/10.3390/metabo13111166 - DOI - PMC - PubMed
-
- Palego L, Betti L, Rossi A, et al.Tryptophan biochemistry: structural, nutritional, metabolic, and medical aspects in humans. J Amino Acids 2016;2016:8952520. https://doi.org/10.1155/2016/8952520 - DOI - PMC - PubMed
-
- Arifuzzaman M, Collins N, Guo CJ, et al.Nutritional regulation of microbiota-derived metabolites: implications for immunity and inflammation. Immunity 2024;57:14–27. https://doi.org/10.1016/j.immuni.2023.12.009 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

