Ancient nitrogenases are ATP dependent
- PMID: 38869277
- PMCID: PMC11253609
- DOI: 10.1128/mbio.01271-24
Ancient nitrogenases are ATP dependent
Abstract
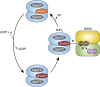
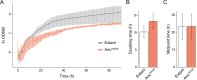
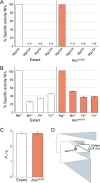
Life depends on a conserved set of chemical energy currencies that are relics of early biochemistry. One of these is ATP, a molecule that, when paired with a divalent metal ion such as Mg2+, can be hydrolyzed to support numerous cellular and molecular processes. Despite its centrality to extant biochemistry, it is unclear whether ATP supported the function of ancient enzymes. We investigate the evolutionary necessity of ATP by experimentally reconstructing an ancestral variant of the N2-reducing enzyme nitrogenase. The Proterozoic ancestor is predicted to be ~540-2,300 million years old, post-dating the Great Oxidation Event. Growth rates under nitrogen-fixing conditions are ~80% of those of wild type in Azotobacter vinelandii. In the extant enzyme, the hydrolysis of two MgATP is coupled to electron transfer to support substrate reduction. The ancestor has a strict requirement for ATP with no other nucleotide triphosphate analogs (GTP, ITP, and UTP) supporting activity. Alternative divalent metal ions (Fe2+, Co2+, and Mn2+) support activity with ATP but with diminished activities compared to Mg2+, similar to the extant enzyme. Additionally, it is shown that the ancestor has an identical efficiency in ATP hydrolyzed per electron transferred to the extant of two. Our results provide direct laboratory evidence of ATP usage by an ancient enzyme.IMPORTANCELife depends on energy-carrying molecules to power many sustaining processes. There is evidence that these molecules may predate the rise of life on Earth, but how and when these dependencies formed is unknown. The resurrection of ancient enzymes provides a unique tool to probe the enzyme's function and usage of energy-carrying molecules, shedding light on their biochemical origins. Through experimental reconstruction, this research investigates the ancestral dependence of a nitrogen-fixing enzyme on the energy carrier ATP, a requirement for function in the modern enzyme. We show that the resurrected ancestor does not have generalist nucleotide specificity. Rather, the ancestor has a strict requirement for ATP, like the modern enzyme, with similar function and efficiency. The findings elucidate the early-evolved necessity of energy-yielding molecules, delineating their role in ancient biochemical processes. Ultimately, these insights contribute to unraveling the intricate tapestry of evolutionary biology and the origins of life-sustaining dependencies.
Keywords: ancestral sequence reconstruction; energy; nitrogen fixation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

