Comparison of SARS-CoV-2 seroprevalence estimates between commercial lab serum specimens and blood donor specimens, United States, September-December 2021
- PMID: 38869287
- PMCID: PMC11302068
- DOI: 10.1128/spectrum.00123-24
Comparison of SARS-CoV-2 seroprevalence estimates between commercial lab serum specimens and blood donor specimens, United States, September-December 2021
Abstract
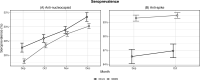
We estimated monthly cross-sectional seroprevalence rates of anti-nucleocapsid (anti-N) and anti-spike (anti-S) antibodies to severe acute respiratory syndrome coronavirus 2 in two U.S. nationwide studies. The nationwide blood donor seroprevalence (NBDS) study included specimens from blood donors, while the nationwide commercial laboratory seroprevalence (NCLS) study included residual serum specimens tested in commercial laboratories for reasons unrelated to the assessment of coronavirus disease 2019 infection. In September-December 2021, specimens collected from both nationwide studies were tested for anti-N antibodies. In September-October 2021, specimens from both studies within a five-state area were tested for anti-S antibodies. We used raking methods to adjust all seroprevalence estimates by the population distribution of key demographics in included states. Seroprevalence estimates of each antibody type were compared across the two studies for specimens drawn in the same U.S. states during the same time period. Our analysis revealed that over a 4-month period, national NCLS monthly anti-N estimates were 0.5-1.9 percentage points higher than NBDS estimates. In contrast, across five states during a 2-month period, NBDS anti-S estimates were 7.6 and 8.2 percentage points higher than NCLS estimates. The observed differences in seroprevalence estimates between the NBDS and NCLS studies may be attributed to variations in the characteristics of the study sample populations, particularly with respect to health status, health behaviors, and vaccination status. These differences should be considered in the interpretation of seroprevalence study results based on blood donors or commercial lab residual specimens.
Importance: This study was the first systematic comparison between two nationwide severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) studies which estimated seroprevalence, or the proportion of the population with antibodies to the virus, using differing convenience sample populations. One study tested blood donor specimens; the other study tested specimens left over from clinical blood tests. The seroprevalence of anti-nucleocapsid and anti-spike antibodies was compared in the same states during the same months with statistical adjustments based on state demographics. Similar anti-nucleocapsid antibody seroprevalence estimates produced by two independent studies using differing convenience samples build confidence in the generalizability of their anti-nucleocapsid findings. Due to high blood donor vaccine rates, blood donor SARS-CoV-2 anti-spike antibody estimates might overestimate general population seroprevalence, an important consideration for interpreting national seroprevalence study results. Furthermore, because laboratory residuals and blood donations are two common sources of specimens for seroprevalence studies, study findings may be informative for other respiratory virus seroepidemiology studies.
Keywords: SARS-CoV-2; anti-nucleocapsid antibody; anti-spike antibody; blood donors; commercial laboratory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Centers for Disease Control and Prevention . 2020. Multistate assessment of SARS-CoV-2 seroprevalence in blood donors. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/blood-bank-seros.... Retrieved 14 Jul 2022.
-
- Centers for Disease Control and Prevention . Commercial laboratory seroprevalence surveys. Available from: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-s.... Retrieved 14 Jul 2022.
-
- Patel EU, Bloch EM, Grabowski MK, Goel R, Lokhandwala PM, Brunker PAR, White JL, Shaz B, Ness PM, Tobian AAR. 2019. Sociodemographic and behavioral characteristics associated with blood donation in the United States: a population-based study, September 2019. Transfusion 59:2899–2907. doi: 10.1111/trf.15415 - DOI - PMC - PubMed
-
- Clarke KEN, Kim Y, Jones J, Lee A, Deng Y, Nycz E, Iachan R, Gundlapalli AV, MacNeil A, Hall A. 2023. Pediatric infection-induced SARS-CoV-2 seroprevalence increases and seroprevalence by type of clinical care—September 2021 to February 2022. J Infect Dis 227:364–370. doi: 10.1093/infdis/jiac423 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous