Feasibility of Endovascular Deep Brain Stimulation of Anterior Nucleus of the Thalamus for Refractory Epilepsy
- PMID: 38869291
- PMCID: PMC11631012
- DOI: 10.1227/ons.0000000000001226
Feasibility of Endovascular Deep Brain Stimulation of Anterior Nucleus of the Thalamus for Refractory Epilepsy
Abstract
Background and objectives: Deep brain stimulation (DBS) has developed into an effective therapy for several disease states including treatment-resistant Parkinson disease and medically intractable essential tremor, as well as segmental, generalized and cervical dystonia, and obsessive-compulsive disorder (OCD). Dystonia and OCD are approved with Humanitarian Device Exemption. In addition, DBS is also approved for the treatment of epilepsy in the anterior nucleus of the thalamus. Although overall considered an effective treatment for Parkinson disease and epilepsy, a number of specific factors determine the treatment success for DBS including careful patient selection, effective postoperative programming of DBS devices and accurate electrode placement. Furthermore, invasiveness of the procedure is a rate limiter for patient adoption. It is desired to explore a less invasive way to deliver DBS therapy.
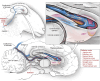
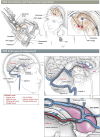
Methods: Here, we report for the first time the direct comparison of endovascular and parenchymal DBS in a triplicate ovine model using the anterior nucleus of the thalamus as the parenchymal target for refractory epilepsy.
Results: Triplicate ovine studies show comparable sensing resolution and stimulation performance of endovascular DBS with parenchymal DBS.
Conclusion: The results from this feasibility study opens up a new frontier for minimally invasive DBS therapy.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc on behalf of Congress of Neurological Surgeons.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

