Manganese uptake by MtsABC contributes to the pathogenesis of human pathogen group A streptococcus by resisting host nutritional immune defenses
- PMID: 38869295
- PMCID: PMC11238556
- DOI: 10.1128/iai.00077-24
Manganese uptake by MtsABC contributes to the pathogenesis of human pathogen group A streptococcus by resisting host nutritional immune defenses
Abstract
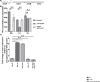
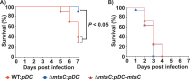
The interplay between host nutritional immune mechanisms and bacterial nutrient uptake systems has a major impact on the disease outcome. The host immune factor calprotectin (CP) limits the availability of essential transition metals, such as manganese (Mn) and zinc (Zn), to control the growth of invading pathogens. We previously demonstrated that the competition between CP and the human pathogen group A streptococcus (GAS) for Zn impacts GAS pathogenesis. However, the contribution of Mn sequestration by CP in GAS infection control and the role of GAS Mn acquisition systems in overcoming host-imposed Mn limitation remain unknown. Using a combination of in vitro and in vivo studies, we show that GAS-encoded mtsABC is a Mn uptake system that aids bacterial evasion of CP-imposed Mn scarcity and promotes GAS virulence. Mn deficiency caused by either the inactivation of mtsC or CP also impaired the protective function of GAS-encoded Mn-dependent superoxide dismutase. Our ex vivo studies using human saliva show that saliva is a Mn-scant body fluid, and Mn acquisition by MtsABC is critical for GAS survival in human saliva. Finally, animal infection studies using wild-type (WT) and CP-/- mice showed that MtsABC is critical for GAS virulence in WT mice but dispensable in mice lacking CP, indicating the direct interplay between MtsABC and CP in vivo. Together, our studies elucidate the role of the Mn import system in GAS evasion of host-imposed metal sequestration and underscore the translational potential of MtsABC as a therapeutic or prophylactic target.
Keywords: manganese; metal uptake; nutritional immunity; pathogenesis; streptococcus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Group A Streptococcus AdcR Regulon Participates in Bacterial Defense against Host-Mediated Zinc Sequestration and Contributes to Virulence.Infect Immun. 2020 Jul 21;88(8):e00097-20. doi: 10.1128/IAI.00097-20. Print 2020 Jul 21. Infect Immun. 2020. PMID: 32393509 Free PMC article.
-
A Critical Role of Zinc Importer AdcABC in Group A Streptococcus-Host Interactions During Infection and Its Implications for Vaccine Development.EBioMedicine. 2017 Jul;21:131-141. doi: 10.1016/j.ebiom.2017.05.030. Epub 2017 Jun 2. EBioMedicine. 2017. PMID: 28596134 Free PMC article.
-
Metal sensing and regulation of adaptive responses to manganese limitation by MtsR is critical for group A streptococcus virulence.Nucleic Acids Res. 2019 Aug 22;47(14):7476-7493. doi: 10.1093/nar/gkz524. Nucleic Acids Res. 2019. PMID: 31188450 Free PMC article.
-
Transition Metal Homeostasis in Streptococcus pyogenes and Streptococcus pneumoniae.Adv Microb Physiol. 2017;70:123-191. doi: 10.1016/bs.ampbs.2017.01.002. Epub 2017 Feb 20. Adv Microb Physiol. 2017. PMID: 28528647 Review.
-
Zinc'ing it out: zinc homeostasis mechanisms and their impact on the pathogenesis of human pathogen group A streptococcus.Metallomics. 2017 Dec 1;9(12):1693-1702. doi: 10.1039/c7mt00240h. Epub 2017 Oct 18. Metallomics. 2017. PMID: 29043347 Free PMC article. Review.
References
-
- Damo SM, Kehl-Fie TE, Sugitani N, Holt ME, Rathi S, Murphy WJ, Zhang Y, Betz C, Hench L, Fritz G, Skaar EP, Chazin WJ. 2013. Molecular basis for manganese sequestration by calprotectin and roles in the innate immune response to invading bacterial pathogens. Proc Natl Acad Sci U S A 110:3841–3846. doi:10.1073/pnas.1220341110 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

