Remote Field Application of Digital Technology for Hearing Assessments in a Cohort of Pediatric Germ Cell Tumor Survivors
- PMID: 38869488
- PMCID: PMC11371521
- DOI: 10.1158/1055-9965.EPI-24-0203
Remote Field Application of Digital Technology for Hearing Assessments in a Cohort of Pediatric Germ Cell Tumor Survivors
Abstract
Background: Childhood cancer survivors treated with platinum-based chemotherapy are at risk of treatment-induced hearing loss. Accurate evaluation of hearing thresholds has historically been limited to clinical audiometry, which is logistically challenging and expensive to include in epidemiologic studies. We evaluated the feasibility of using a remote, tablet-based hearing assessment in a cohort of pediatric germ cell tumor survivors treated with platinum-based chemotherapy.
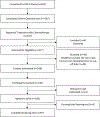
Methods: Survivors from the GCT Outcomes and Late effects Data (GOLD) study were recruited to the pilot study (n = 100). Study personnel conducted remote hearing assessments of standard and extended high frequency thresholds using validated tablet-based audiometry (SHOEBOX, Inc.). T tests and Wilcoxon rank-sum tests evaluated differences in assessment characteristics between children and adults. Agreement between self-reported and measured hearing loss was calculated using Cohen κ.
Results: We were able to reach 136/168 (81%) eligible participants, of which 100 (74%) agreed to participate. Successful completion of the remote hearing assessment was high [97%; 20 children (ages 7-17), 77 adults (ages 18-31)]. The mean assessment length was 37.6 minutes, and the mean turnaround time was 8.3 days. We observed hearing loss at standard frequencies in 21% of participants. Agreement between self-reported and measured hearing loss was significant (P value = 1.41 × 10-7), with 83.5% concordance.
Conclusions: Hearing loss measured using the remote assessment aligns with self-reporting and rates of hearing loss reported in the literature for this population.
Impact: Remote application of tablet-based audiometry is a feasible and efficacious method for measuring hearing in epidemiologic studies with participants spread across large geographic areas.
©2024 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Thyagarajan B, Nelson HH, Poynter JN, Prizment AE, Roesler MA, Cassidy E, et al. Field Application of Digital Technologies for Health Assessment in the 10,000 Families Study. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2020. Apr;29(4):744–51. - PubMed
-
- Knight KRG, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol Off J Am Soc Clin Oncol. 2005. Dec 1;23(34):8588–96. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases