EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary
- PMID: 38869512
- PMCID: PMC11519095
- DOI: 10.1007/s00125-024-06196-3
EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary
Erratum in
-
Publisher Correction: EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD): Executive Summary.Diabetologia. 2024 Nov;67(11):2608. doi: 10.1007/s00125-024-06258-6. Diabetologia. 2024. PMID: 39352523 Free PMC article. No abstract available.
Abstract
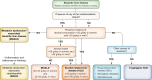
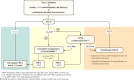
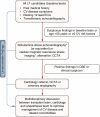
Metabolic dysfunction-associated steatotic liver disease (MASLD), previously termed non-alcoholic fatty liver disease (NAFLD), is defined as steatotic liver disease (SLD) in the presence of one or more cardiometabolic risk factor(s) and the absence of harmful alcohol intake. The spectrum of MASLD includes steatosis, metabolic dysfunction-associated steatohepatitis (MASH, previously NASH), fibrosis, cirrhosis and MASH-related hepatocellular carcinoma (HCC). This joint EASL-EASD-EASO guideline provides an update on definitions, prevention, screening, diagnosis and treatment for MASLD. Case-finding strategies for MASLD with liver fibrosis, using non-invasive tests, should be applied in individuals with cardiometabolic risk factors, abnormal liver enzymes and/or radiological signs of hepatic steatosis, particularly in the presence of type 2 diabetes or obesity with additional metabolic risk factor(s). A stepwise approach using blood-based scores (such as the fibrosis-4 index [FIB-4]) and, sequentially, imaging techniques (such as transient elastography) is suitable to rule-out/in advanced fibrosis, which is predictive of liver-related outcomes. In adults with MASLD, lifestyle modification-including weight loss, dietary changes, physical exercise and discouraging alcohol consumption-as well as optimal management of comorbidities-including use of incretin-based therapies (e.g. semaglutide, tirzepatide) for type 2 diabetes or obesity, if indicated-is advised. Bariatric surgery is also an option in individuals with MASLD and obesity. If locally approved and dependent on the label, adults with non-cirrhotic MASH and significant liver fibrosis (stage ≥2) should be considered for a MASH-targeted treatment with resmetirom, which demonstrated histological effectiveness on steatohepatitis and fibrosis with an acceptable safety and tolerability profile. No MASH-targeted pharmacotherapy can currently be recommended for the cirrhotic stage. Management of MASH-related cirrhosis includes adaptations of metabolic drugs, nutritional counselling, surveillance for portal hypertension and HCC, as well as liver transplantation in decompensated cirrhosis.
Keywords: Diabetes; Glucagon-like peptide; Hepatocellular carcinoma; Liver fibrosis; MASH; MASLD; NAFLD; NASH; Non-invasive tests; Resmetirom.
© 2024. The Author(s).
Conflict of interest statement
Luca Valenti has disclosed speaking engagements with Viatris, Novo Nordisk, and GSK; consulting roles with Novo Nordisk, Pfizer, Boehringer Ingelheim, Resalis, and MSD; and received unrestricted grant support from Gilead. Elisabetta Bugianesi serves as a consultant for Novo Nordisk, Boehringer Ingelheim, Eli Lilly, Pfizer, Bristol-Myers Squibb, and MSD. Amalia Gastaldelli has acted as a consultant for Boehringer Ingelheim, Eli Lilly and Company, and Metadeq Diagnostics; participated in advisory boards for Boehringer Ingelheim, Merck Sharp & Dohme, Novo Nordisk, Metadeq Diagnostics, and Pfizer; and received speaker's honorarium or other fees from Eli Lilly and Company, Merck Sharp & Dohme, Novo Nordisk, and Pfizer. Frank Tacke’s lab has received research funding from Gilead, AstraZeneca, and MSD (funding to the institution); he has received honoraria for consulting or lectures from AstraZeneca, Gilead, AbbVie, BMS, Boehringer, Intercept, Falk, Inventiva, MSD, GSK, Orphalan, Merz, Pfizer, Alnylam, Novo Nordisk, Sanofi, and Novartis. Michael Roden has participated in consulting for and/or scientific advisory boards of BMS, Boehringer Ingelheim Pharma, Echosens, Eli Lilly, Madrigal, MSD, Novo Nordisk, as well as in clinical trials supported by Boehringer Ingelheim, Novo Nordisk, and Nutricia/Danone. Paul Horn received grants from Novo Nordisk (through the University of Birmingham) and MSD (through Charité - Universitätsmedizin Berlin); speaking fees from Orphalan; and travel support from IPSEN. Hannele Yki-Järvinen declares no conflicts of interest regarding the present work. Shira Zelber-Sagi received travel support for attending a conference and honoraria for a lecture presentation in this conference by AbbVie, a one-time consulting fee by SIEMENS, and holds an unpaid leadership role as Chair-elect of the EASL public health committee. Vincent Wong has served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Intercept, Inventiva, Novo Nordisk, Pfizer, Sagimet Biosciences, TARGET PharmaSolutions, and Visirna; a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk, and Unilab; received a research grant from Gilead Sciences, and is a co-founder of Illuminatio Medical Technology. Gema Frühbeck received payment of honoraria for attendance to Advisory Boards from Lilly, and Novo Nordisk as well as payment of honoraria for lectures as Member of the OPEN Spain Initiative. Sven Francque holds a senior clinical investigator fellowship from the Research Foundation Flanders (FWO) and has acted as consultant for Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer, Roche. He has acted as consultant for Abbvie, Actelion, Aelin Therapeutics, AgomAb, Aligos Therapeutics, Allergan, Alnylam, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristoll-Meyers Squibb, CSL Behring, Coherus, Echosens, Dr. Falk Pharma, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Genflow Biosciences, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, PRO.MED.CS Praha, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, Mursla Bio, NGM Bio, Novartis, Novo Nordisk, Promethera, Roche, Siemens Healthineers, and lectured for for Abbvie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk, Promethera, Siemens. Dror Dicker received grants, personal fees, and nonfinancial support from NovoNordisk and Eli Lilly, and personal fees and nonfinancial support from Boehringer Ingelheim outside the submitted work. Vlad Ratziu serves as a consultant for Novo-Nordisk, Madrigal, Boehringer-Ingelheim, GSK, and 89 Bio; and has a grant to institution from MSD. Fritz Schick declares no conflict of interest pertaining to this article. Roberto Vettor has received honoraria from Novo Nordisk, Eli Lilly and AstraZeneca, and has served on advisory boards for Novo Nordisk and Eli Lilly.
Figures


References
-
- OCEBM Levels of Evidence Working Group. “The Oxford 2011 Levels of Evidence”. Oxford Centre for Evidence-Based Medicine. 2011. Available from: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-e.... Accessed 27 Jan 2023

