Emotional Awareness and Expression Therapy vs Cognitive Behavioral Therapy for Chronic Pain in Older Veterans: A Randomized Clinical Trial
- PMID: 38869899
- PMCID: PMC11177167
- DOI: 10.1001/jamanetworkopen.2024.15842
Emotional Awareness and Expression Therapy vs Cognitive Behavioral Therapy for Chronic Pain in Older Veterans: A Randomized Clinical Trial
Abstract
Importance: Chronic pain is common and disabling in older adults, and psychological interventions are indicated. However, the gold standard approach, cognitive-behavioral therapy (CBT), produces only modest benefits, and more powerful options are needed.
Objectives: To evaluate whether emotional awareness and expression therapy (EAET) is superior to CBT for treatment of chronic pain among predominantly male older veterans and whether higher baseline depression, anxiety, or posttraumatic stress disorder (PTSD) symptoms-key targets of EAET-moderate treatment response.
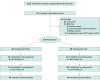
Design, setting, and participants: This 2-arm randomized clinical trial was conducted from May 16, 2019, to September 14, 2023, in the US Department of Veterans Affairs Greater Los Angeles Healthcare System. The trial included a racially and ethnically diverse group of veterans aged 60 to 95 years with at least 3 months of musculoskeletal pain.
Interventions: Emotional awareness and expression therapy or CBT, conducted concurrently, each presented as one 90-minute individual session followed by eight 90-minute group sessions.
Main outcomes and measures: The primary outcome was Brief Pain Inventory pain severity (range, 0 to 10) from baseline to posttreatment (week 10, primary end point) and 6-month follow-up. Secondary outcomes included Patient Reported Outcomes Institute Measurement System Anxiety, Depression, Fatigue, General Life Satisfaction (NIH Toolbox), Pain Interference, and Sleep Disturbance Short Forms, Patient Global Impression of Change (PGIC), and Satisfaction with Therapy and Therapist Scale-Revised. A subset of participants completed the PTSD Checklist for Diagnostic and Statistical Manual of Mental Disorders (Fifth Edition). All analyses were for the intention-to-treat population and included all randomized participants.
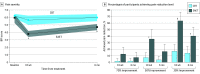
Results: Among 126 randomized participants (66 in the EAET group and 60 in the CBT group; mean [SD] age, 71.9 [5.9] years; 116 [92%] male), 111 (88%) completed posttreatment, and 104 (82%) completed the 6-month follow-up. The EAET was superior to CBT for the primary outcome of reduction in pain severity at posttreatment (estimate, -1.59 [95% CI, -2.35 to -0.83]; P < .001) and follow-up (estimate, -1.01 [95% CI, -1.78 to -0.24]; P = .01). A greater percentage of participants in EAET vs CBT had clinically significant (at least 30%) pain reduction (63% vs 17%; odds ratio, 21.54 [95% CI, 4.66-99.56]; P < .001) at posttreatment. In addition, EAET was superior to CBT on 50% pain reduction (35% vs 7%; odds ratio, 11.77 [95% CI, 2.38-58.25]; P = .002), anxiety (estimate, -2.49 [95% CI, -4.30 to -0.68]; P = .006), depression (estimate, -3.06 [95% CI, -5.88 to -0.25]; P = .03), general life satisfaction (estimate, 1.23 [95% CI, 0.36-2.10]; P = .005), PTSD symptoms (estimate, -4.39 [95% CI, -8.44 to -0.34]; P = .03), PGIC score (estimate, 1.46 [95% CI, 0.77-2.15]; P < .001), and global treatment satisfaction (estimate, 0.28 [95% CI, 0.12-0.45]; P < .001) at posttreatment. Higher baseline depression (estimate, -1.55 [95% CI, -0.37 to 2.73]; P < .001), anxiety (estimate, -1.53 [95% CI, -2.19 to -0.88]; P < .001), and PTSD symptoms (estimate, -1.69 [95% CI, -2.96 to -0.42]; P = .009) moderated greater reduction in pain severity after EAET but not CBT.
Conclusions and relevance: The results of this randomized clinical trial suggest that EAET may be a preferred intervention for medically and psychiatrically complex patients with pain. The societal burden of chronic pain could be improved by further incorporating the principles of EAET into mainstream clinical pain medicine.
Trial registration: ClinicalTrials.gov Identifier: NCT03918642.
Conflict of interest statement
Figures

Comment in
-
Addressing the Emotional Body in Patients With Chronic Pain.JAMA Netw Open. 2024 Jun 3;7(6):e2417340. doi: 10.1001/jamanetworkopen.2024.17340. JAMA Netw Open. 2024. PMID: 38869904 No abstract available.

