Neutrophil glucose flux as a therapeutic target in antiphospholipid syndrome
- PMID: 38869951
- PMCID: PMC11290966
- DOI: 10.1172/JCI169893
Neutrophil glucose flux as a therapeutic target in antiphospholipid syndrome
Abstract
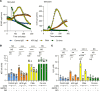
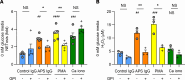
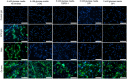
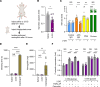
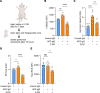
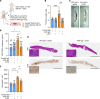
Neutrophil hyperactivity and neutrophil extracellular trap release (NETosis) appear to play important roles in the pathogenesis of the thromboinflammatory autoimmune disease known as antiphospholipid syndrome (APS). The understanding of neutrophil metabolism has advanced tremendously in the past decade, and accumulating evidence suggests that a variety of metabolic pathways guide neutrophil activities in health and disease. Our previous work characterizing the transcriptome of APS neutrophils revealed that genes related to glycolysis, glycogenolysis, and the pentose phosphate pathway (PPP) were significantly upregulated. Here, we found that neutrophils from patients with APS used glycolysis more avidly than neutrophils from people in the healthy control group, especially when the neutrophils were from patients with APS with a history of microvascular disease. In vitro, inhibiting either glycolysis or the PPP tempered phorbol myristate acetate- and APS IgG-induced NETosis, but not NETosis triggered by a calcium ionophore. In mice, inhibiting either glycolysis or the PPP reduced neutrophil reactive oxygen species production and suppressed APS IgG-induced NETosis ex vivo. When APS-associated thrombosis was evaluated in mice, inhibiting either glycolysis or the PPP markedly suppressed thrombosis and circulating NET remnants. In summary, these data identify a potential role for restraining neutrophil glucose flux in the treatment of APS.
Keywords: Autoimmune diseases; Autoimmunity; Glucose metabolism; Neutrophils.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

