Adrenal gland macrophages regulate glucocorticoid production through Trem2 and TGF-β
- PMID: 38869957
- PMCID: PMC11383592
- DOI: 10.1172/jci.insight.174746
Adrenal gland macrophages regulate glucocorticoid production through Trem2 and TGF-β
Abstract
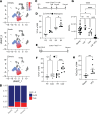
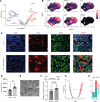
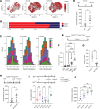
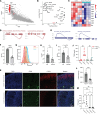
Glucocorticoid synthesis by adrenal glands (AGs) is regulated by the hypothalamic-pituitary-adrenal axis to facilitate stress responses when the host is exposed to stimuli. Recent studies implicate macrophages as potential steroidogenic regulators, but the molecular mechanisms by which AG macrophages exert such influence remain unclear. In this study, we investigated the role of AG macrophages in response to cold challenge or atherosclerotic inflammation as physiologic models of acute or chronic stress. Using single-cell RNA sequencing, we observed dynamic AG macrophage polarization toward classical activation and lipid-associated phenotypes following acute or chronic stimulation. Among transcriptional alterations induced in macrophages, triggering receptor expressed on myeloid cells 2 (Trem2) was highlighted because of its upregulation following stress. Conditional deletion of macrophage Trem2 revealed a protective role in stress responses. Mechanistically, Trem2 deletion led to increased AG macrophage death, abolished the TGF-β-producing capacity of AG macrophages, and resulted in enhanced glucocorticoid production. In addition, enhanced glucocorticoid production was replicated by blockade of TGF-β signaling. Together, these observations suggest that AG macrophages restrict steroidogenesis through Trem2 and TGF-β, which opens potential avenues for immunotherapeutic interventions to resolve stress-related disorders.
Keywords: Cytokines; Endocrinology; Immunology; Macrophages.
Conflict of interest statement
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

