Exclusion of sulfide:quinone oxidoreductase from mitochondria causes Leigh-like disease in mice by impairing sulfide metabolism
- PMID: 38870029
- PMCID: PMC11290971
- DOI: 10.1172/JCI170994
Exclusion of sulfide:quinone oxidoreductase from mitochondria causes Leigh-like disease in mice by impairing sulfide metabolism
Abstract
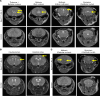
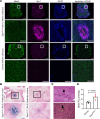
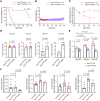
Leigh syndrome is the most common inherited mitochondrial disease in children and is often fatal within the first few years of life. In 2020, mutations in the gene encoding sulfide:quinone oxidoreductase (SQOR), a mitochondrial protein, were identified as a cause of Leigh syndrome. Here, we report that mice with a mutation in the gene encoding SQOR (SqorΔN/ΔN mice), which prevented SQOR from entering mitochondria, had clinical and pathological manifestations of Leigh syndrome. SqorΔN/ΔN mice had increased blood lactate levels that were associated with markedly decreased complex IV activity and increased hydrogen sulfide (H2S) levels. Because H2S is produced by both gut microbiota and host tissue, we tested whether metronidazole (a broad-spectrum antibiotic) or a sulfur-restricted diet rescues SqorΔN/ΔN mice from developing Leigh syndrome. Daily treatment with metronidazole alleviated increased H2S levels, normalized complex IV activity and blood lactate levels, and prolonged the survival of SqorΔN/ΔN mice. Similarly, a sulfur-restricted diet normalized blood lactate levels and inhibited the development of Leigh syndrome. Taken together, these observations suggest that mitochondrial SQOR is essential to prevent systemic accumulation of H2S. Metronidazole administration and a sulfur-restricted diet may be therapeutic approaches to treatment of patients with Leigh syndrome caused by mutations in SQOR.
Keywords: Metabolism; Mitochondria; Mouse models; Neurological disorders; Therapeutics.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

