A highly reproducible and efficient method for retinal organoid differentiation from human pluripotent stem cells
- PMID: 38870053
- PMCID: PMC11194494
- DOI: 10.1073/pnas.2317285121
A highly reproducible and efficient method for retinal organoid differentiation from human pluripotent stem cells
Abstract
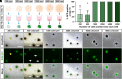
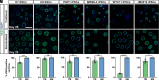
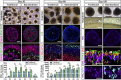
Human pluripotent stem cell (hPSC)-derived retinal organoids are three-dimensional cellular aggregates that differentiate and self-organize to closely mimic the spatial and temporal patterning of the developing human retina. Retinal organoid models serve as reliable tools for studying human retinogenesis, yet limitations in the efficiency and reproducibility of current retinal organoid differentiation protocols have reduced the use of these models for more high-throughput applications such as disease modeling and drug screening. To address these shortcomings, the current study aimed to standardize prior differentiation protocols to yield a highly reproducible and efficient method for generating retinal organoids. Results demonstrated that through regulation of organoid size and shape using quick reaggregation methods, retinal organoids were highly reproducible compared to more traditional methods. Additionally, the timed activation of BMP signaling within developing cells generated pure populations of retinal organoids at 100% efficiency from multiple widely used cell lines, with the default forebrain fate resulting from the inhibition of BMP signaling. Furthermore, given the ability to direct retinal or forebrain fates at complete purity, mRNA-seq analyses were then utilized to identify some of the earliest transcriptional changes that occur during the specification of these two lineages from a common progenitor. These improved methods also yielded retinal organoids with expedited differentiation timelines when compared to traditional methods. Taken together, the results of this study demonstrate the development of a highly reproducible and minimally variable method for generating retinal organoids suitable for analyzing the earliest stages of human retinal cell fate specification.
Keywords: organoid; retina; stem cell.
Conflict of interest statement
Competing interests statement:J.H. and J.S.M. have filed a patent related to the methodology described in this paper.
Figures



References
-
- Nakano T., et al. , Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

