Opioids and the Gastrointestinal Tract: The Role of Peripherally Active µ-Opioid Receptor Antagonists in Modulating Intestinal Permeability
- PMID: 38870087
- PMCID: PMC11446513
- DOI: 10.14309/ajg.0000000000002887
Opioids and the Gastrointestinal Tract: The Role of Peripherally Active µ-Opioid Receptor Antagonists in Modulating Intestinal Permeability
Abstract
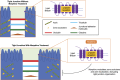
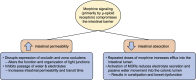
Opioid receptors are found throughout the gastrointestinal tract, including the large intestine. Many patients treated with opioids experience opioid-induced constipation (OIC). Laxatives are not effective in most patients, and in those who do initially respond, the efficacy of laxatives generally diminishes over time. In addition, OIC does not spontaneously resolve for most patients. However, complications of opioids extend far beyond simply slowing gastrointestinal transit. Opioid use can affect intestinal permeability through a variety of mechanisms. Toll-like receptors are a crucial component of innate immunity and are tightly regulated within the gut epithelium. Pathologic µ-opioid receptor (MOR) and toll-like receptor signaling, resulting from chronic opioid exposure, disrupts intestinal permeability leading to potentially harmful bacterial translocation, elevated levels of bacterial toxins, immune activation, and increased cytokine production. Peripherally active MOR antagonists, including methylnaltrexone, are effective at treating OIC. Benefits extend beyond simply blocking the MOR; these agents also act to ameliorate opioid-induced disrupted intestinal permeability. In this review, we briefly describe the physiology of the gastrointestinal epithelial border and discuss the impact of opioids on gastrointestinal function. Finally, we consider the use of peripherally active MOR antagonists to treat disrupted intestinal permeability resulting from opioid use and discuss the potential for improved morbidity and mortality in patients treated with methylnaltrexone for opioid-induced bowel disorders.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures




Similar articles
-
Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care.Cochrane Database Syst Rev. 2018 Jun 5;6(6):CD006332. doi: 10.1002/14651858.CD006332.pub3. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2022 Sep 15;9:CD006332. doi: 10.1002/14651858.CD006332.pub4. PMID: 29869799 Free PMC article. Updated.
-
Pharmacological prevention and treatment of opioid-induced constipation in cancer patients: A systematic review and meta-analysis.Cancer Treat Rev. 2024 Apr;125:102704. doi: 10.1016/j.ctrv.2024.102704. Epub 2024 Mar 1. Cancer Treat Rev. 2024. PMID: 38452708
-
Efficacy of methylnaltrexone for the treatment of opiod-induced constipation: a meta-analysis and systematic review.Postgrad Med. 2016;128(3):282-9. doi: 10.1080/00325481.2016.1149017. Epub 2016 Feb 23. Postgrad Med. 2016. PMID: 26839023
-
Efficacy of pharmacological therapies for the treatment of opioid-induced constipation: systematic review and meta-analysis.Am J Gastroenterol. 2013 Oct;108(10):1566-74; quiz 1575. doi: 10.1038/ajg.2013.169. Epub 2013 Jun 11. Am J Gastroenterol. 2013. PMID: 23752879
-
Mu-opioid antagonists for opioid-induced bowel dysfunction in people with cancer and people receiving palliative care.Cochrane Database Syst Rev. 2022 Sep 15;9(9):CD006332. doi: 10.1002/14651858.CD006332.pub4. Cochrane Database Syst Rev. 2022. PMID: 36106667 Free PMC article.
Cited by
-
Effects of opium on cholesterol metabolism in rats fed normal and high-fat/high-cholesterol diet.Toxicol Rep. 2025 Mar 26;14:102014. doi: 10.1016/j.toxrep.2025.102014. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40230514 Free PMC article.
-
Transdermal fentanyl induced paralytic intestinal obstruction in advanced liver cancer: a case report.Front Pharmacol. 2025 Mar 5;16:1550296. doi: 10.3389/fphar.2025.1550296. eCollection 2025. Front Pharmacol. 2025. PMID: 40110133 Free PMC article.
-
Treatment of Opioid-Induced Constipation: Inducing Laxation and Understanding the Risk of Gastrointestinal Perforation.J Clin Gastroenterol. 2025 Jul 1;59(6):491-496. doi: 10.1097/MCG.0000000000002185. J Clin Gastroenterol. 2025. PMID: 40434810 Free PMC article. Review.
-
Gut sensory neurons as regulators of neuro-immune-microbial interactions: from molecular mechanisms to precision therapy for IBD/IBS.J Neuroinflammation. 2025 Jul 2;22(1):172. doi: 10.1186/s12974-025-03500-9. J Neuroinflammation. 2025. PMID: 40605050 Free PMC article. Review.
-
Sugammadex and Gastric Emptying: Individualized Patient Effects.Anesth Analg. 2025 Jul 31;141(4):e53-4. doi: 10.1213/ANE.0000000000007663. Online ahead of print. Anesth Analg. 2025. PMID: 40742889 Free PMC article. No abstract available.
References
-
- Dahlhamer JM, Connor EM, Bose J, et al. Prescription opioid use among adults with chronic pain: United States, 2019. Natl Health Stat Report 2021;(162):1–9. - PubMed
-
- Kalso E, Edwards JE, Moore AR, et al. Opioids in chronic non-cancer pain: Systematic review of efficacy and safety. Pain 2004;112(3):372–80. - PubMed
-
- Bell TJ, Panchal SJ, Miaskowski C, et al. The prevalence, severity, and impact of opioid-induced bowel dysfunction: Results of a US and European patient survey (PROBE 1). Pain Med 2009;10(1):35–42. - PubMed
-
- Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016;150(6):1393–407.e5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

