Effectiveness and safety of azvudine in COVID-19: A systematic review and meta-analysis
- PMID: 38870134
- PMCID: PMC11175417
- DOI: 10.1371/journal.pone.0298772
Effectiveness and safety of azvudine in COVID-19: A systematic review and meta-analysis
Abstract
Objective: The aim of this study was to assess the effectiveness and safety of azvudine in treating coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2).
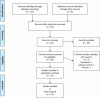
Methods: A search was carried out in PubMed, Cochrane Library, Web of Science, medRxiv, and Google Scholar until October 20, 2023. The Cochrane risk of bias tools were used to assess the quality of included studies. Comprehensive Meta-Analysis software was used to analyze data.
Results: Twenty-one studies including 10,011 patients were examined. The meta-analysis results showed that azvudine and standard of care/placebo (SOC/PBO) were significantly different concerning mortality rate (risk ratio [RR] = 0.48, 95% confidence interval [CI]: 0.40 to 0.57) and negative polymerase chain reaction (PCR) conversion time (standard mean difference = - 0.75, 95% CI: -1.29 to-0.21). However, the two groups did not show significant differences concerning hospital stay, intensive care unit (ICU) admission, and need for mechanical ventilation (P > 0.05). On the other hand, azvudine and nirmatrelvir-ritonavir were significantly different in mortality rate (RR = 0.73, 95% CI: 0.58 to 0.92), ICU admission (RR = 0.41, 95% CI: 0.21 to 0.78), and need for mechanical ventilation (RR = 0.67, 95% CI: 0.51 to 0.89), but the two treatments were not significantly different in negative PCR conversion time, and hospital stay (P > 0.05). The incidence of adverse events between groups was not significant (P > 0.05). The certainty of evidence was rated as low or moderate.
Conclusions: The antiviral effectiveness of azvudine against SARS-COV-2 is questionable with regard to the certainty of evidence. Further research should be conducted to establish the effectiveness and safety of azvudine in COVID-19.
Copyright: © 2024 Amani, Amani. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
-
- Cegolon L, Pol R, Simonetti O, Larese Filon F, Luzzati R. Molnupiravir, Nirmatrelvir/Ritonavir, or Sotrovimab for High-Risk COVID-19 Patients Infected by the Omicron Variant: Hospitalization, Mortality, and Time until Negative Swab Test in Real Life. Pharmaceuticals. 2023;16(5):721. doi: 10.3390/ph16050721 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

