Clinical impact of rapid molecular diagnostic tests in patients presenting with viral respiratory symptoms: A systematic literature review
- PMID: 38870136
- PMCID: PMC11175541
- DOI: 10.1371/journal.pone.0303560
Clinical impact of rapid molecular diagnostic tests in patients presenting with viral respiratory symptoms: A systematic literature review
Abstract
Background: Molecular tests can detect lower concentrations of viral genetic material over a longer period of respiratory infection than antigen tests. Delays associated with central laboratory testing can result in hospital-acquired transmission, avoidable patient admission, and unnecessary use of antimicrobials, all which may lead to increased cost of patient management. The aim of this study was to summarize comparisons of clinical outcomes associated with rapid molecular diagnostic tests (RMDTs) versus other diagnostic tests for viral respiratory infections.
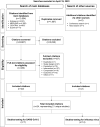
Methods: A systematic literature review (SLR) conducted in April 2023 identified studies evaluating clinical outcomes of molecular and antigen diagnostic tests for patients suspected of having respiratory viral infections.
Results: The SLR included 21 studies, of which seven and 14 compared RMDTs (conducted at points of care or at laboratories) to standard (non-rapid) molecular tests or antigen tests to detect SARS-CoV-2 and influenza, respectively. In studies testing for SARS-CoV-2, RMDTs led to reductions in time to test results versus standard molecular tests (range of the reported medians: 0.2-3.8 hours versus 4.3-35.9 hours), with similar length of emergency department stay (3.2-8 hours versus 3.7-28.8 hours). Similarly, in studies testing for influenza, RMDTs led to reductions in time to test results versus standard molecular tests (1-3.5 hours versus 18.2-29.2 hours), with similar length of emergency department stay (3.7-11 hours versus 3.8-11.9 hours). RMDTs were found to decrease exposure time of uninfected patients, rate of hospitalization, length of stay at the hospitals, and frequency of unnecessary antiviral and antibacterial therapy, while improving patient flow, compared to other tests.
Conclusions: Compared to other diagnostic tests, RMDTs improve clinical outcomes, test turnaround time, and stewardship by decreasing unnecessary use of antibiotics and antivirals. They also reduce hospital admission and length of stay, which may, in turn, reduce unnecessary exposure of patients to hospital-acquired infections and their associated costs.
Copyright: © 2024 Mojebi et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: AM and SK are employees and shareholders of PRECISIONheor, which received funding from Cepheid for this work. PW and BH are employees of PRECISIONheor, which received funding from Cepheid for this work. JGC and AB are employees and shareholders of Cepheid, which provided funding for this work.
Figures
Similar articles
-
Impact of molecular point-of-care testing on clinical management and in-hospital costs of patients suspected of influenza or RSV infection: a modeling study.J Med Virol. 2019 Aug;91(8):1408-1414. doi: 10.1002/jmv.25479. Epub 2019 Apr 14. J Med Virol. 2019. PMID: 30950066 Free PMC article.
-
Point of care testing of Influenza A/B and RSV in an adult respiratory assessment unit is associated with improvement in isolation practices and reduction in hospital length of stay.J Med Microbiol. 2020 May;69(5):697-704. doi: 10.1099/jmm.0.001187. Epub 2020 Apr 6. J Med Microbiol. 2020. PMID: 32250239 Free PMC article.
-
Modelling of hypothetical SARS-CoV-2 point-of-care tests on admission to hospital from A&E: rapid cost-effectiveness analysis.Health Technol Assess. 2021 Mar;25(21):1-68. doi: 10.3310/hta25210. Health Technol Assess. 2021. PMID: 33764295 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
-
Rapid Molecular Tests for Influenza, Respiratory Syncytial Virus, and Other Respiratory Viruses: A Systematic Review of Diagnostic Accuracy and Clinical Impact Studies.Clin Infect Dis. 2019 Sep 13;69(7):1243-1253. doi: 10.1093/cid/ciz056. Clin Infect Dis. 2019. PMID: 30689772 Free PMC article.
Cited by
-
Are We Getting Closer to the "Cholesterol" for Chronic Respiratory Disease?Am J Respir Crit Care Med. 2024 Sep 12;211(1):6-7. doi: 10.1164/rccm.202407-1480ED. Online ahead of print. Am J Respir Crit Care Med. 2024. PMID: 39265184 Free PMC article. No abstract available.
-
Innovations in MALDI-TOF Mass Spectrometry: Bridging modern diagnostics and historical insights.Open Life Sci. 2025 Jul 18;20(1):20251136. doi: 10.1515/biol-2025-1136. eCollection 2025. Open Life Sci. 2025. PMID: 40688408 Free PMC article. Review.
-
Diagnostic tools in respiratory medicine (Review).Biomed Rep. 2025 May 8;23(1):112. doi: 10.3892/br.2025.1990. eCollection 2025 Jul. Biomed Rep. 2025. PMID: 40420977 Free PMC article. Review.
References
-
- World Health Organization. Global influenza strategy 2019–2030. Cataloguing-in-Publication (CIP) data. CIP data are available at http://apps.who.int/iris. 2019 [Available from: https://apps.who.int/iris/bitstream/handle/10665/311184/9789241515320-en....
-
- World Health Organization. WHO Coronavirus (COVID‐19) Dashboard [Available from: https://covid19.who.int.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous