Mechanobiological Modulation of In Vitro Astrocyte Reactivity Using Variable Gel Stiffness
- PMID: 38870483
- PMCID: PMC11234334
- DOI: 10.1021/acsbiomaterials.4c00229
Mechanobiological Modulation of In Vitro Astrocyte Reactivity Using Variable Gel Stiffness
Abstract
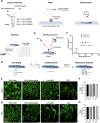
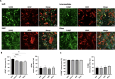
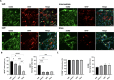
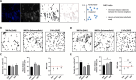
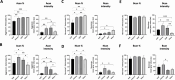
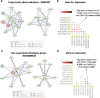
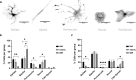
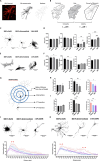
After traumatic brain injury, the brain extracellular matrix undergoes structural rearrangement due to changes in matrix composition, activation of proteases, and deposition of chondroitin sulfate proteoglycans by reactive astrocytes to produce the glial scar. These changes lead to a softening of the tissue, where the stiffness of the contusion "core" and peripheral "pericontusional" regions becomes softer than that of healthy tissue. Pioneering mechanotransduction studies have shown that soft substrates upregulate intermediate filament proteins in reactive astrocytes; however, many other aspects of astrocyte biology remain unclear. Here, we developed a platform for the culture of cortical astrocytes using polyacrylamide (PA) gels of varying stiffness (measured in Pascal; Pa) to mimic injury-related regions in order to investigate the effects of tissue stiffness on astrocyte reactivity and morphology. Our results show that substrate stiffness influences astrocyte phenotype; soft 300 Pa substrates led to increased GFAP immunoreactivity, proliferation, and complexity of processes. Intermediate 800 Pa substrates increased Aggrecan+, Brevican+, and Neurocan+ astrocytes. The stiffest 1 kPa substrates led to astrocytes with basal morphologies, similar to a physiological state. These results advance our understanding of astrocyte mechanotransduction processes and provide evidence of how substrates with engineered stiffness can mimic the injury microenvironment.
Keywords: astrocyte morphology; astrogliosis; glial scar; matrix stiffness; polyacrylamide gels.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Mechanically induced reactive gliosis causes ATP-mediated alterations in astrocyte stiffness.J Neurotrauma. 2009 May;26(5):789-97. doi: 10.1089/neu.2008-0727. J Neurotrauma. 2009. PMID: 19331521 Free PMC article.
-
Astrogliosis in a dish: substrate stiffness induces astrogliosis in primary rat astrocytes.RSC Adv. 2016;6(41):34447-34457. doi: 10.1039/C5RA25916A. Epub 2016 Mar 17. RSC Adv. 2016. PMID: 32742641 Free PMC article.
-
Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures.Biophys J. 2006 Apr 15;90(8):3012-8. doi: 10.1529/biophysj.105.073114. Epub 2006 Feb 3. Biophys J. 2006. PMID: 16461391 Free PMC article.
-
Connexins in Astrocyte Migration.Front Pharmacol. 2020 Jan 15;10:1546. doi: 10.3389/fphar.2019.01546. eCollection 2019. Front Pharmacol. 2020. PMID: 32009957 Free PMC article. Review.
-
Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system.Curr Opin Cell Biol. 2015 Feb;32:121-30. doi: 10.1016/j.ceb.2015.02.004. Epub 2015 Mar 2. Curr Opin Cell Biol. 2015. PMID: 25726916 Review.
Cited by
-
Enhanced Response of ZnO Nanorod-Based Flexible MEAs for Recording Ischemia-Induced Neural Activity in Acute Brain Slices.Nanomaterials (Basel). 2025 Jul 30;15(15):1173. doi: 10.3390/nano15151173. Nanomaterials (Basel). 2025. PMID: 40801712 Free PMC article.
-
Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome.Int J Mol Sci. 2025 Apr 2;26(7):3298. doi: 10.3390/ijms26073298. Int J Mol Sci. 2025. PMID: 40244122 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Mortality data on CDC WONDER.; National Center for Health Statistics. https://wonder.cdc.gov/mcd.html (accessed 2022–08–17).
-
- Wanner I. B.; Anderson M. A.; Song B.; Levine J.; Fernandez A.; Gray-Thompson Z.; Ao Y.; Sofroniew M. V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. 10.1523/JNEUROSCI.2121-13.2013. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
